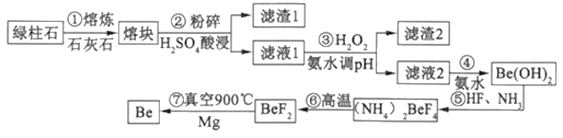
��Ŀ����
����Ŀ����һ���������ܱ������з�����ѧ��Ӧ��2A(g) ![]() B(g)��C(g)��������A����ʼŨ��Ϊ1.0 mol��L��1������B��C����ʼŨ��Ϊ0ʱ������A��Ũ�ȡ�ת�����Լ�����B��Ũ���淴Ӧʱ��ı仯�����и�ͼ��ʾ��
B(g)��C(g)��������A����ʼŨ��Ϊ1.0 mol��L��1������B��C����ʼŨ��Ϊ0ʱ������A��Ũ�ȡ�ת�����Լ�����B��Ũ���淴Ӧʱ��ı仯�����и�ͼ��ʾ��

�������й�˵������ȷ����
A. ����I������IIʱ�ķ�Ӧ�¶Ȳ�ͬ��ѹǿ��ͬ
B. ����Iʱ����δʹ�ô���������IIʱ����ʹ���˴���
C. ����IIIʱ��ƽ�������У�����C��Ũ�ȵ���0.6 mol��L��1
D. ����IV������II��Ƚϣ�����IVʱ������С���������
���𰸡�A
��������
�������ͼ���֪ͼI��ͼIIƽ��ʱA��Ũ������ͬ�ģ����ﵽƽ���ʱ�䲻ͬ������Ϊ��Ӧǰ��������䣬��˵����Ӧ�¶���ͬ��ѹǿ����ͬ��ʹ���˴�����A����ȷ��B��ȷ������IIIʱA��ƽ��ת������0.6����������C��Ũ����0.3mol/L��C����ȷ������IV������II��Ƚϣ�ƽ��ʱ��״̬����ͬ�ģ��Ҵﵽƽ���ʱ��Ҳ����ͬ�ģ�����D����ȷ����ѡB��

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ