��Ŀ����
7������ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ���H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
������ʯ�к������IJⶨ
�ٰ���ͼ��װ���������װ�õ������ԣ�
�ڽ�5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
�۴���˵����ܿڴ����ϵػ���ͨH2����Cװ�ó��ڴ�H2�鴿��ȼA���ƾ���
�ܳ�ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ��ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ�����
��2����ķ�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ24%��
��1�����������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2��
��2����������õ��IJ����������ձ�������������ͷ�ιܡ�250mL����ƿ��
��3�������йز���IJ�����˵����ȷ����df��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���Һ��ϴ
e���ζ������У��۾�ע�ӵζ�����Һ��仯
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ���
��4�����ζ�����������0.5000mol•L-1KI��Һ20.00mL��������ʯ�����İٷֺ���Ϊ70%��
���ɢ���������������ʯ������������Ļ�ѧʽΪFe5O6 ��
���� ��1��B�еļ�ʯ���������û���Ӧ���ɵ�ˮ�ģ�Ϊ�˷�ֹ�����ɷֶ�ʵ���Ӱ�죬Ҫ��һ��װ�����տ����е�ˮ�Լ�������̼��
��2����Ӧ��װ��B����1.35g������������������Ӧ���������������ֵ�����Ը��ݲ����������㣻
��1����п��Խ�ˮ�е�������ߣ�
��2������ϡ��Һ�������һ���������Һ��ѡ����������ش�
��3�����ݵζ������Լ��ζ������е�ʵ��������֪ʶ���ش��жϣ�
��4������Ԫ���غ�ͻ�ѧ��Ӧ����ʽ���м��㣻
������Ԫ��������������Ԫ�������������������������Ļ�ѧʽ��
��� �⣺��1����ʵ���У���������������Ӧ���ɽ�������ˮ�����ݹ��������ı仯���������ĺ�����B���ĸ�������������ղ�����ˮ����������Cװ��Ҫ��ֹ��ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ�����
�ʴ�Ϊ����ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ�����
��2����ķ�Ӧ��װ��B����1.35g�����ݷ�Ӧ��ʵ�ʣ����ӵ���ˮ�����������������غ㶨�ɣ���������ʯ�����İٷֺ����ǣ�$\frac{\frac{1.35g}{18g/mol}��16g/mol}{5.0g}$��100%=24%��
�ʴ�Ϊ��24%��
��1��������ʯ�м������ᣬ����Ӧ���������������Һ�������ڹ�����������Һ�������������������к���Խ����������ܽ�ȣ�������Һ���ܽ�Ĺ�����Cl2��
�ʴ�Ϊ��������Һ���ܽ�Ĺ�����Cl2��
��2����ԭ��Һϡ�͵�250mL����Ҫʹ�õIJ������������У��ձ�������������ͷ�ιܡ�250mL����ƿ����ȱ��250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
��3��a����ˮΪ��ɫ������������Ҳ�ǻ�ɫ��Һ���ζ����������ָʾ������a����
b���ζ������У����������Ժ͵����ӷ�����Ӧ�����������Ӻ͵ⵥ�ʣ��ⵥ������������Һ��ʾ��ɫ������ȷ���Ƿ�ﵽ�ζ��յ㣬��b����
c���ζ���������ˮϴ�Ӻ�����ñ�Һ��ϴ����c����
d����ƿ����Ҫ�ô���Һ��ϴ����d��ȷ��
e���ζ������У��۾�ע����ƿ����ɫ�ı仯����e����
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ�������f��ȷ��
�ʴ�Ϊ��df��
��4�����ݷ�Ӧ�ķ���ʽΪ2Fe3++2I-=2Fe2++I2��֪�����ĵĵ����������������ʵ�����ȣ�n��Fe3+��=n��KI��������0.4000mol•L-1��0.025L=c��Fe3+����0.02L�����c��Fe3+��=0.5mol•L-1��������Ԫ�صİٷֺ���Ϊ��$\frac{0.5mol/L��0.25L��56g/mol}{10g}$��100%=70%��
�ʴ�Ϊ��70%��
����������������70%����Ԫ�ص�����������24%������100g����ʯ�У���Ԫ�ص�������70g����Ԫ��������24g����Ԫ�غ���Ԫ�ص����ʵ�����Ϊ��$\frac{70}{56}$��$\frac{24}{16}$=5��6��
����������Ļ�ѧʽΪ��Fe5O6��
�ʴ�Ϊ��Fe5O6 ��
���� ���⿼����̽������ʯ����Ԫ�غ���Ԫ�صĺ����ķ����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬���ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ��������������������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ�Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۣ�

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�| A�� |  �����������ƹ�������� �����������ƹ�������� | |
| B�� |  ����е����ϴ�Ļ���Һ������ ����е����ϴ�Ļ���Һ������ | |
| C�� |  ���������ռ����� ���������ռ����� | |
| D�� |  ���뻥�����ܵ�����Һ�� ���뻥�����ܵ�����Һ�� |
| A�� | Na+��Fe2+��NO3-��Cl- | B�� | Na+��K+��NO3-��Cl- | ||
| C�� | Na+��K+��Al��OH��4-��Cl- | D�� | NH4+��K+��SO42-��HCO3- |
| A�� | K+��OH-��CO32 | B�� | CO32-��OH-��Cl- | C�� | K+��H+��Cl- | D�� | Cu2+��H+��Cl- |
| A�� | ��λ�����Һ�����������ʵ����ʵ������������ʵ���Ũ�� | |
| B�� | ����Ħ���������22.4L mol- | |
| C�� | Ħ����������������Է��������������ԭ������ | |
| D�� | ���ʵ����������ʵ����� |

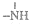 ��
��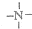 һ�������¶����Ժ�H+��ϣ���N4��������H+��Ӧ���ɵ����ӵĻ�ѧʽΪN4H44+��
һ�������¶����Ժ�H+��ϣ���N4��������H+��Ӧ���ɵ����ӵĻ�ѧʽΪN4H44+��