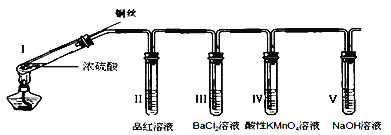
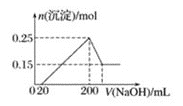
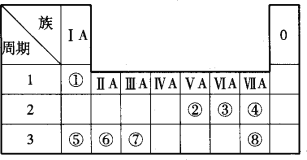
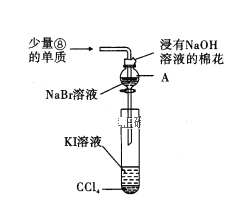
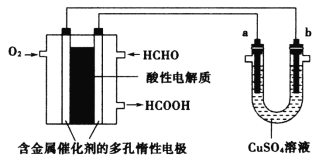
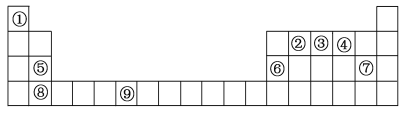
��Ŀ����
����Ŀ��A��B��C��D����Ԫ�أ�AԪ���γɵ�-2�������ӱȺ��ĺ����������8 ����BԪ�ص�һ��������Ϊ����ɫ���壬�ù�����������������A�ĵ��ʡ�CΪԭ�Ӻ���12�����ӵĵ�IIA�����Ԫ�أ���2.4g C��������ˮ��Ӧʱ���ڱ�״���·ų�����2.24L��D��M������7�����ӡ�
��1����Ԫ�ط��ţ�A______��C_______��D_______��
��2��B��C��D����������ˮ����Ļ�ѧʽ����Ϊ_______�����м�����ǿ����________��
��3���Ƚ�D����̬�⻯����HF���ȶ��ԣ�__________��
���𰸡��� þ �� NaOH��Mg��OH��2��HClO4 NaOH HCl��HF
��������
��A��B��C��D����Ԫ�أ�AԪ���γɵ�-2�������ӱȺ��ĺ����������8������A��������Ϊ2+8-2=8����AΪ��Ԫ�أ�BԪ�ص�һ��������Ϊ����ɫ���壬��BΪNaԪ�أ�CΪԭ�Ӻ�����12�����ӵĶ��۽�������2.4gC��������ˮ��Ӧʱ���ڱ�״̬�·ų�����2.24L����C�����ԭ������Ϊx�����ݵ���ת���غ���![]() �����x=24����C��������Ϊ24-12=12����CΪMgԪ�أ�D��M������7�����ӣ���DΪClԪ�ء�
�����x=24����C��������Ϊ24-12=12����CΪMgԪ�أ�D��M������7�����ӣ���DΪClԪ�ء�
��1��������������֪��A������C��þ��D���ȣ�
�ʴ�Ϊ������þ���ȣ�
��2��B��C��D����������ˮ���ﻯѧʽ�ֱ�ΪNaOH��Mg��OH��2��HClO4��ͬ���������������Ӧˮ����ļ��Դ��������������м�����ǿ����NaOH���ʴ�Ϊ��NaOH��Mg��OH��2��HClO4��NaOH��
��3��D����̬�⻯��ΪHCl���ǽ�����F��Cl�����⻯���ȶ���HCl��HF���ʴ�Ϊ��HCl��HF��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�