��Ŀ����
�Ȼ���(BN)������һ���������ϳɲ��ϡ�����ɰ(Na2B4O7)�����ط�Ӧ���Եõ�������Na2B4O7+ 2CO(NH2)2��4BN+Na2O +4H2O+2CO2��������Ҫ��ش��������⣺
��1����ɷ�Ӧ�������Ԫ���У���һ������������___________________��
��2�����ط���(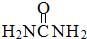 )��
)��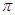 ����
���� ����Ŀ֮��Ϊ__________�����ط����д���ͬһƽ���ԭ�������_______����
����Ŀ֮��Ϊ__________�����ط����д���ͬһƽ���ԭ�������_______����
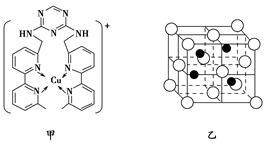
��3�����ط���һ���������γ������Ρ������ӡ����ṹ��ͼ�����������ӡ������ط��Ӽ���Ҫͨ��ʲô��������ϡ���________________(��1��)��
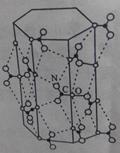
��4��ͼʾ�������ӡ������᷽����һ��ͨ������ֱ���������Ӹպ��ܽ���ͨ�������γɡ������ӡ��İ�̨�֧���������в������ռ�����ϴ�������롰ͨ������������һ���ʿ���ʵ��ֱ��������֧�������ķ��롣
��ֱ�����������ܽ���ͨ��ʱ��ͨ��ʲô�������롰�����ӡ���ϣ��Ӷ��γ� �������ӡ�������?��___________________��
���������ʿ���ͨ�����ء������ӡ����з������____________��
A������Ͷ��� B��������춡�� C��������������� D�Ȼ��ƺ��Ȼ���
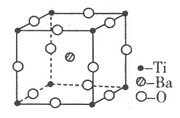
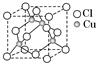
��5��BN������a��B�������ͣ���a��BN�ṹ��ʯī���ơ�B��BN�ṹ����ʯ���ơ�
��aһBN������Nԭ���ӻ���ʽ��____________________��
��B��BN�����У�ÿ����ԭ���γ�________�����ۼ�����Щ���ۼ��У���________��Ϊ��λ����
��1����ɷ�Ӧ�������Ԫ���У���һ������������___________________��
��2�����ط���(
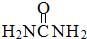 )��
)��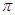 ����
���� ����Ŀ֮��Ϊ__________�����ط����д���ͬһƽ���ԭ�������_______����
����Ŀ֮��Ϊ__________�����ط����д���ͬһƽ���ԭ�������_______������3�����ط���һ���������γ������Ρ������ӡ����ṹ��ͼ�����������ӡ������ط��Ӽ���Ҫͨ��ʲô��������ϡ���________________(��1��)��

��4��ͼʾ�������ӡ������᷽����һ��ͨ������ֱ���������Ӹպ��ܽ���ͨ�������γɡ������ӡ��İ�̨�֧���������в������ռ�����ϴ�������롰ͨ������������һ���ʿ���ʵ��ֱ��������֧�������ķ��롣
��ֱ�����������ܽ���ͨ��ʱ��ͨ��ʲô�������롰�����ӡ���ϣ��Ӷ��γ� �������ӡ�������?��___________________��
���������ʿ���ͨ�����ء������ӡ����з������____________��
A������Ͷ��� B��������춡�� C��������������� D�Ȼ��ƺ��Ȼ���
��5��BN������a��B�������ͣ���a��BN�ṹ��ʯī���ơ�B��BN�ṹ����ʯ���ơ�
��aһBN������Nԭ���ӻ���ʽ��____________________��
��B��BN�����У�ÿ����ԭ���γ�________�����ۼ�����Щ���ۼ��У���________��Ϊ��λ����
��1��N��2�֣�
��2��1�U7��2�֣� 6��2�֣�
��3�������2�֣�
��4���ٷ��»�����1�֣� ��B��1�֣�
��5����sp2��1�֣� ��4 1����1�֣���2�֣�
��2��1�U7��2�֣� 6��2�֣�
��3�������2�֣�
��4���ٷ��»�����1�֣� ��B��1�֣�
��5����sp2��1�֣� ��4 1����1�֣���2�֣�
�����������1����ɷ�Ӧ�������Ԫ��Na��B��O��C��N��H���ǽ�����Խǿ��һ������Խ�����ڵ�Ԫ�ص�2p������Ӵ��ڰ����״̬���ȶ���ǿ�����Ե�Ԫ�صĵ�һ�����ܴ�����Ԫ�صģ�����ɷ�Ӧ�������Ԫ���У���һ������������N��
��2����������
 ����˫������1��
����˫������1�� ����1��
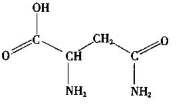
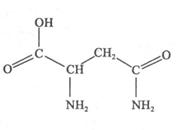
����1�� �����ɵģ����Ը������ط��ӵĽṹ��ʽ
�����ɵģ����Ը������ط��ӵĽṹ��ʽ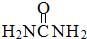 ��֪��������
��֪�������� ����
���� ����Ŀ֮��Ϊ1�U7������̼��˫����ƽ���νṹ��2����ԭ��λ�����ƽ���ϡ����ڰ����������νṹ����˰����е�2����ԭ�������1��λ�����ƽ���ϣ�������ط����д���ͬһƽ���ԭ�������6����
����Ŀ֮��Ϊ1�U7������̼��˫����ƽ���νṹ��2����ԭ��λ�����ƽ���ϡ����ڰ����������νṹ����˰����е�2����ԭ�������1��λ�����ƽ���ϣ�������ط����д���ͬһƽ���ԭ�������6������3�������γɵľ����Ƿ��Ӿ��壬����Ԫ���뵪Ԫ�ؾ����γ���������ԡ������ӡ������ط��Ӽ���Ҫͨ�������������ϡ�
��4���������γɵľ��������Ƿ��Ӿ��壬���ͨ�����Ӽ������������»����롰�����ӡ���ϣ��Ӷ��γ� �������ӡ������
�ڹؽ��������֪������ʵ��ֱ��������֧�������ķ��룬������Ͷ��鶼����֧��������������������֧�����Ȼ��ƺ��Ȼ��ز�����������˲���ʵ�ַ��룬������춡��ֱ���ֱ��������֧������������ʵ�ַ��룬��ѡB��
��5����a��BN�ṹ��ʯī���ƣ���ʯī�Dz�״�ṹ��̼ԭ����sp2�ӻ�������aһBN������Nԭ���ӻ���ʽ��sp2�ӻ���
��B��BN�ṹ����ʯ���ƣ����ʯ��ÿ��̼ԭ���γ�4�����ۼ�������B��BN�����У�ÿ����ԭ��Ҳ�γ�4�����ۼ�������Bԭ�ӵ�������������3����ֻ���γ�3�����ۼ��������Щ���ۼ��У���1��Ϊ��λ����

��ϰ��ϵ�д�
�����Ŀ
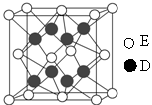
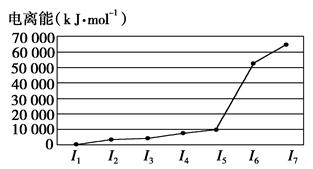
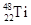 ��
��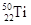 ����ԭ�ӣ����ǻ���Ϊ ���ѻ�̬ԭ�ӵĵ����Ų�ʽΪ ��
����ԭ�ӣ����ǻ���Ϊ ���ѻ�̬ԭ�ӵĵ����Ų�ʽΪ ��