��Ŀ����
A��B��C��D��E����Ԫ�أ�AԪ�ص�����������������ԭ��������ͬ��B�Ļ�̬ԭ�Ӻ�����3��������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ��CԪ�صĵ�������ͼ��ʾ��DԪ�صļ۵��ӹ���Ϊnsnnpn��2��E�ǵ�4���ڵĹ���Ԫ�أ�Ѫ�쵰���е�EԪ����BD�γɵ���λ������D2�γɵ���λ��ǿ��E������BD�γɵ������E��BD��5�������³�Һ̬���۵�Ϊ��20.5�棬�е�Ϊ103 �棬�����ڷǼ����ܼ���
CԪ�صĵ�����
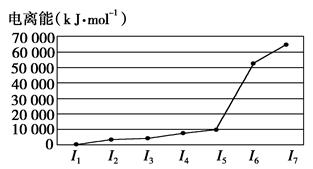
��1��E��BD��5��������________��������ͣ���
��2��AԪ�غ�BԪ����ɵĻ��������֮��________����ܡ����ܡ����γ������
��3����̬Eԭ�ӵĵ����Ų�ʽΪ___________________________________ ��
��4��B��C��D����Ԫ�صĵ縺���ɴ�С��˳����________����Ԫ�ط��ţ���
��5��C2��B2A2�ķ����и��ݵ������ص��ķ�ʽ��ͬ���������Ĺ��ۼ�������________��
��6����֪ԭ�����͵�������ͬ�����еȵ����壬�ȵ�����Ľṹ���ơ�����
�±����ݣ�˵��BD���ӱ�C2���ӻ��õ�ԭ��_____________________________��
CԪ�صĵ�����

��1��E��BD��5��������________��������ͣ���
��2��AԪ�غ�BԪ����ɵĻ��������֮��________����ܡ����ܡ����γ������
��3����̬Eԭ�ӵĵ����Ų�ʽΪ___________________________________ ��
��4��B��C��D����Ԫ�صĵ縺���ɴ�С��˳����________����Ԫ�ط��ţ���
��5��C2��B2A2�ķ����и��ݵ������ص��ķ�ʽ��ͬ���������Ĺ��ۼ�������________��
��6����֪ԭ�����͵�������ͬ�����еȵ����壬�ȵ�����Ľṹ���ơ�����
�±����ݣ�˵��BD���ӱ�C2���ӻ��õ�ԭ��_____________________________��
| | X��Y | X��Y | X��Y |
| BD�ļ���/kJ��mol��1 | 357.7 | 798.9 | 1 071.9 |
| C2�ļ���/kJ��mol��1 | 154.8 | 418.4 | 941.7 |
��1�����Ӿ���
��2������
��3��1s22s22p63s23p63d64s2
��4��O��N��C
��5���Ҽ��ͦм�
��6��CO�ж��ѵ�һ���м����ĵ�������273 kJ����N2�ж��ѵ�һ���м����ĵ�������523.3 kJ��С��CO�ĵ�һ���м��������ѣ����CO�ϻ���
��2������
��3��1s22s22p63s23p63d64s2
��4��O��N��C
��5���Ҽ��ͦм�
��6��CO�ж��ѵ�һ���м����ĵ�������273 kJ����N2�ж��ѵ�һ���м����ĵ�������523.3 kJ��С��CO�ĵ�һ���м��������ѣ����CO�ϻ���
��������Ϣ����֪A��H��B��C��C��N��DΪO��EΪFe����1�������Fe��CO��5�����³�Һ̬�������ڷǼ����ܼ�����Fe��CO��5����Ϊ���Ӿ��塣��2��CԪ�صķǽ����Խ�������C��HԪ���γɵĻ�������û���������3���ԡ���4��C��N��O��ͬ���ڵ�Ԫ�أ���縺������ԭ��������������������ߵ縺���ɴ�С��˳���ǣ�O��N��C����5��N2��C2H2�ķ����ж������Ĺ��ۼ��ЦҼ��ͦм�����N��N��C��C�ж�������һ���Ҽ��������м�������6��CO��N2�ǵȵ����壬�ṹ���ƣ���CO�ж��ѵ�һ���м����ĵ�����Ϊ��1 071.9��798.9��273 kJ����N2�ж��ѵ�һ���м����ĵ�����Ϊ��941.7��418.4��523.3 kJ���ɼ�CO�ĵ�һ���м������ѣ����CO��N2���á�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
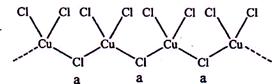

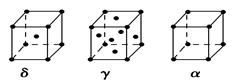
 ����
���� ����Ŀ֮��Ϊ ��
����Ŀ֮��Ϊ ��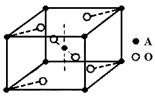
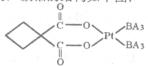
 ��
�� ��
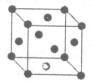
��
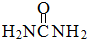 )��
)��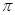 ����
����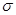 ����Ŀ֮��Ϊ__________�����ط����д���ͬһƽ���ԭ�������_______����
����Ŀ֮��Ϊ__________�����ط����д���ͬһƽ���ԭ�������_______����