��Ŀ����
�춬����(�ṹ��ͼ)��«���к����ḻ��������������������Ĺ�Ч��
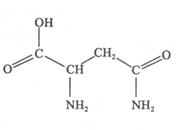
��1���춬��������Ԫ���У� (��Ԫ������)Ԫ�ػ�̬ԭ�Ӻ���δ�ɶԵ�������࣬�춬������̼ԭ�ӵ��ӻ���������� �֡�
��2��H2S��H2Se�IJ����Աȼ�����
��H2Se�ľ�������Ϊ �����еĹ��ۼ�����Ϊ ��
��H2S�ļ��Ǵ���H2Se��ԭ�����Ϊ ��
��3����֪��(Mo)λ�ڵ�������VIB�壬�⡢�����̵IJ��ֵ��������±���ʾ
A�� (��Ԫ�ط���)��B�ļ۵����Ų�ʽΪ ��

��1���춬��������Ԫ���У� (��Ԫ������)Ԫ�ػ�̬ԭ�Ӻ���δ�ɶԵ�������࣬�춬������̼ԭ�ӵ��ӻ���������� �֡�
��2��H2S��H2Se�IJ����Աȼ�����
| ��ѧʽ | ������nm | ���� | �е㣯�� |
| H2S | 1.34 | 92.3o | һ60.75 |
| H2Se | 1.47 | 91.0o | һ41.50 |
��H2Se�ľ�������Ϊ �����еĹ��ۼ�����Ϊ ��
��H2S�ļ��Ǵ���H2Se��ԭ�����Ϊ ��
��3����֪��(Mo)λ�ڵ�������VIB�壬�⡢�����̵IJ��ֵ��������±���ʾ
| ��� | I5��kJ��mol-1 | I6��kJ��mol-1 | I7��kJ��mol-1 | I8��kJ��mol-1 |
| A | 6990 | 9220 | 11500 | 18770 |
| B | 6702 | 8745 | 15455 | 17820 |
| C | 5257 | 6641 | 12125 | 13860 |
A�� (��Ԫ�ط���)��B�ļ۵����Ų�ʽΪ ��
��1������1�֣� 2 ��1�֣�
��2���ٷ��Ӿ��壨2�֣� ���Լ���2�֣�
�� ����S�ĵ縺��ǿ��Se���γɵĹ��õ��ӶԳ������Ǵ�2�֣�
��3��Mn��2�֣� 3d54s1��2�֣�
��2���ٷ��Ӿ��壨2�֣� ���Լ���2�֣�
�� ����S�ĵ縺��ǿ��Se���γɵĹ��õ��ӶԳ������Ǵ�2�֣�
��3��Mn��2�֣� 3d54s1��2�֣�
�����������1���춬��������̼���⡢����������Ԫ�أ���̬ԭ�Ӻ���δ�ɶԵ���������Ϊ��Ԫ�ء�
��2����H2Se�ڳ���ʱΪ�ɷǽ���Ԫ���γɵ���̬�⻯�Ϊ���Ӿ��壻H��SeΪ��ͬԪ�أ��γɵĹ��ۼ�Ϊ���Լ���
��S��SeΪͬ����Ԫ�أ�Sλ�ڵ������ڡ�Seλ�ڵ������ڣ�S�ĵ縺��ǿ��Se���γɵĹ��õ��ӶԳ���������H2S�ļ��Ǵ���H2Se��
��3��Aԭ�� I5��I6�� I7�� I8��������Aԭ��ʧȥ��8�����Ӷ��Ǽ۵��ӣ�����AΪMnԪ�أ�B��Cԭ��I7��I6���˺ܶ࣬˵��B��Cԭ�ӵļ۵���Ϊ6��������������B����C��˵��BΪ�������ڵ�Cr��CΪ��(Mo)λ�ڵ������ڣ�Cr�ļ۵����Ų�ʽΪ��3d54s1

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
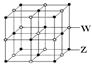
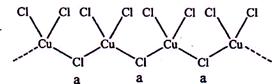
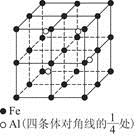
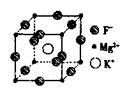


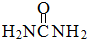 )��
)��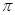 ����
����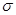 ����Ŀ֮��Ϊ__________�����ط����д���ͬһƽ���ԭ�������_______����
����Ŀ֮��Ϊ__________�����ط����д���ͬһƽ���ԭ�������_______����