��Ŀ����
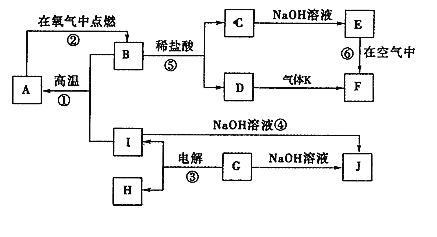
����Ŀ���л�������A��H��ת����ϵ������ʾ��
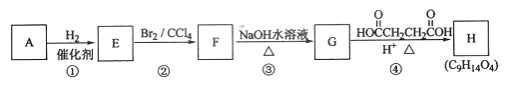
����A��֧����ֻ��һ�������ţ�����Է���������65��75֮�䣬1 mol A��ȫȼ������7 mol��������ش��������⣺
��1��A�Ľṹ��ʽ��________��
��2����Fת��ΪG�Ļ�ѧ����ʽ��_______________________��
��3��G������Ʒ�Ӧ�ܷų����壬��Gת��ΪH�Ļ�ѧ����ʽ��_______________��
��4���ٵķ�Ӧ������___________���۵ķ�Ӧ������____________��
��5������B��A��ͬ���칹�壬�����е�����̼ԭ�ӹ�ƽ�棬����⻯����Ϊ�����飬д��B��һ���ܵĽṹ��ʽ_______________________________��
��6��CҲ��A��һ��ͬ���칹�壬����һ�ȴ���ֻ��һ�֣������������칹����C�Ľṹ��ʽΪ__________________________��
���𰸡���CH3��2CHC��CH ��CH3��2CHCHBrCH2Br+2NaOH![]()
![]() +2NaBr
+2NaBr ![]() +HOOCCH2CH2COOH
+HOOCCH2CH2COOH![]()
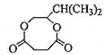 +2H2O �ӳɷ�Ӧ����ԭ��Ӧ�� ȡ����Ӧ CH3CH=CHCH=CH2����д����˳�����칹�壩��CH3CH2C��CCH3
+2H2O �ӳɷ�Ӧ����ԭ��Ӧ�� ȡ����Ӧ CH3CH=CHCH=CH2����д����˳�����칹�壩��CH3CH2C��CCH3 ![]()
��������
��1��G�붡���ᷴӦ����H������H�ķ���ʽ��֪��G�����к���5��̼ԭ�ӣ�������A�к���5��̼ԭ�ӣ�Aȼ�յķ���ʽΪC5Hy+(5+![]() )O2
)O2![]() 5CO2+
5CO2+![]() H2O��1 mol A��ȫȼ������7 mol������5+
H2O��1 mol A��ȫȼ������7 mol������5+![]() =7�����y=8��A�ķ���ʽ��C5H8������A��֧����ֻ��һ�������ţ�����A�Ľṹ��ʽ��(CH3)2CHC��CH��������3������1����Ȳ��
=7�����y=8��A�ķ���ʽ��C5H8������A��֧����ֻ��һ�������ţ�����A�Ľṹ��ʽ��(CH3)2CHC��CH��������3������1����Ȳ��
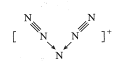
��2������A�Ľṹ��ʽ��֪��A��H2�����ӳɷ�Ӧ����E��E��Br2/CCl4�����ӳɷ�Ӧ����F����E�Ľṹ��ʽ��(CH3)2CHCH=CH2����F�Ľṹ��ʽ��(CH3)2CHCHBrCH2Br��F��NaOHˮ��Һ����ˮ�ⷴӦ����G��G�Ľṹ��ʽΪ![]() ��Fת��ΪG�Ļ�ѧ����ʽ�ǣ�CH3��2CHCHBrCH2Br+2NaOH
��Fת��ΪG�Ļ�ѧ����ʽ�ǣ�CH3��2CHCHBrCH2Br+2NaOH![]()
![]() +2NaBr��
+2NaBr��
��3��G����H�ķ�Ӧ��������Ӧ������H�ķ���ʽ���жϣ�HӦ���ǻ��������Է�Ӧ�ķ���ʽ��![]() +HOOCCH2CH2COOH
+HOOCCH2CH2COOH![]()
 +2H2O��
+2H2O��
��4����Ӧ�٢۵ķ�Ӧ���ͷֱ��Ǽӳɷ�Ӧ����ԭ��Ӧ����ȡ����Ӧ����ˮ�ⷴӦ����
��5������B��A��ͬ���칹�壬�����е�����̼ԭ�ӹ�ƽ�棬����⻯����Ϊ�����飬��B�Ľṹ��ʽ��CH3CH=CHCH=CH2����д����˳�����칹�壩��CH3CH2C��CCH3��
��6��CҲ��A��һ��ͬ���칹�壬����һ�ȴ���ֻ��һ�֣�˵�������ǶԳƵģ����C�Ľṹ��ʽ��![]() ��
��

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�