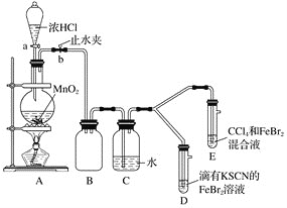
ЬтФПФкШн
ЁОЬтФПЁПNa2S2O4ЁЄ2H2OЪЧШОСЯЙЄвЕжаГЃгУЕФЛЙдМСЃЌЫзГЦБЃЯеЗлЫќПЩШмгкЧтбѕЛЏФЦШмвКВЂЮШЖЈДцдкЃЌФбШмгкввДМЃЌЪмШШвзЗжНтЃЌжЦБИБЃЯеЗлЕФСїГЬШчЯТ:
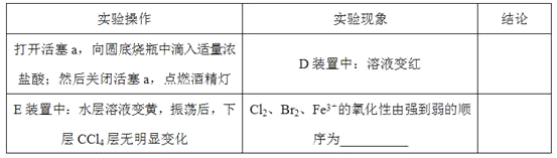
ЛиД№ЯТСаЮЪЬт:
(1)БЃЯеЗлЕФжЦБИЙ§ГЬвЊдкЮобѕЬѕМўЯТНјааЃЌдвђЪЧ__________
(2)ШєгУNa2SO3ЙЬЬхКЭЫсЗДгІжЦБИSO2ЦјЬхЃЌЯТСазюЪЪвЫбЁгУЕФЫсЪЧ____(ЬюБъКХ)
A.ХЈбЮЫс B.ЯЁЯѕЫс C.70%СђЫс D.98%СђЫс
(3)ВНжшЂкашвЊПижЦЮТЖШдк35ЁцЕФдвђЪЧ__________вбжЊpH>11ЪБЃЌZn(OH) 2зЊЛЏЮЊ![]() ЃЌЮЊСЫЪЙZn2+ГСЕэЭъШЋ,дђМгШыЧтбѕЛЏФЦШмвКЕїНкШмвКЕФpHЗЖЮЇЪЧ______________(25ЁцЪБKsp[Zn(OH)2]=1.0ЁС1017ЃЌ35ЁцЪБKspгыKwЕФБфЛЏПЩКіТд)
ЃЌЮЊСЫЪЙZn2+ГСЕэЭъШЋ,дђМгШыЧтбѕЛЏФЦШмвКЕїНкШмвКЕФpHЗЖЮЇЪЧ______________(25ЁцЪБKsp[Zn(OH)2]=1.0ЁС1017ЃЌ35ЁцЪБKspгыKwЕФБфЛЏПЩКіТд)
(4)ВНжшЂлжаМгШывЛЖЈСПNaClЙЬЬхЕФФПЕФЪЧ____
(5)ВНжшЂмЕФВйзїЪЧ__________ОИЩдяЕУNa2S2O4ЁЄ2H2OбљЦЗ
(6)ЮЊСЫВтЖЈNa2S2O4ЁЄ2H2OбљЦЗДПЖШЃЌШЁmgбљЦЗШмНтдкзуСПЕФМзШЉШмвКжаЃЌХфжЦГЩ100.00mLШмвКШЁ10.00mLШмвКгкзЖаЮЦПжаЃЌгУcmol/LЕтБъзМвКЕЮЖЈжСжеЕуЃЌЯћКФБъзМвКЕФЬхЛ§ЮЊVmLВтЖЈЙ§ГЬжаЃЌЗЂЩњЕФЗДгІЃКNa2S2O4+2HCHO+H2OЈTNaHSO3CH2O+NaHSO2CH2OЃЌNaHSO2CH2O+2I2+2H2OЈTNaHSO4+HCHO+4HIЃЌдђбљЦЗжаNa2S2O4ЁЄ2H2OЕФДПЖШЮЊ____
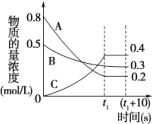
ЁОД№АИЁПБЃЯеЗлОпгаЛЙдадЃЌШнвзБЛбѕЦјбѕЛЏ C ЮТЖШЕЭгк35ЁцЃЌЗДгІЫйТЪЬЋТ§ЃЌЮТЖШЬЋИпЃЌNa2S2O4ШнвзЗжНт [8-11) діДѓc(Na+)ЃЌЪЙЦНКтNa2S2O4(s)![]() 2Na+(aq)+ S2O42-(aq)ФцЯђвЦЖЏЃЌБугкNa2S2O4ОЇЬхЮіГі Й§ТЫЃЌгУввДМЯДЕг2-3ДЮ
2Na+(aq)+ S2O42-(aq)ФцЯђвЦЖЏЃЌБугкNa2S2O4ОЇЬхЮіГі Й§ТЫЃЌгУввДМЯДЕг2-3ДЮ ![]()
ЁОНтЮіЁП
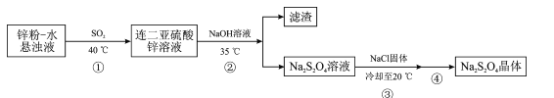
ZnКЭSO2ЗДгІЩњГЩZnS2O4ЃЌПижЦЮТЖШдк35ЁцЃЌЪЙZnS2O4КЭNaOHЗДгІЩњГЩNa2S2O4КЭZn(OH)2ГСЕэЃЌЭЈЙ§Й§ТЫГ§ШЅZn(OH)2ЃЌЕУNa2S2O4ШмвКЃЌNa2S2O4ШмвКжаМгШыNaClдіДѓNa+ЕФХЈЖШЃЌЭЌЪБНЕЮТЪЙNa2S2O4ОЇЬхЮіГіЃЌОнДЫНтД№ЁЃ
(1)БЃЯеЗлОпгаЛЙдадЃЌдкгабѕЬѕМўЯТЗДгІШнвзБЛбѕЛЏЃЌЫљвдЃЌБЃЯеЗлЕФжЦБИЙ§ГЬвЊдкЮобѕЬѕМўЯТНјааЃЌЙЪД№АИЮЊЃКБЃЯеЗлОпгаЛЙдадЃЌШнвзБЛбѕЦјбѕЛЏЃЛ
(2)AЃЎХЈбЮЫсвзЛгЗЂЃЌЛсЛьгаHClЃЌAВЛТњзуЬтвтЃЛ
BЃЎЯЁЯѕЫсгаЧПбѕЛЏадЃЌгыNa2SO3ЗДгІЕУВЛЕНSO2ЃЌBВЛТњзуЬтвтЃЛ
CЃЎ70%СђЫсКЭNa2SO3ФмНЯПьВњЩњSO2ЃЌCТњзуЬтвтЃЛ
DЃЎ98%ЕФСђЫсХЈЖШЬЋДѓЃЌКмФбЕчРыГіЧтРызгЃЌЗДгІВњЩњЖўбѕЛЏСђЕФЫйТЪБШНЯТ§ЃЌDВЛТњзуЬтвтЃЛ
ЙЪД№АИЮЊЃКCЃЛ
(3)ЮТЖШЕЭгк35ЁцЃЌЗДгІЫйТЪЬЋТ§ЃЌЮТЖШЬЋИпЃЌNa2S2O4ШнвзЗжНтЃЌЫљвдВНжшЂкашвЊПижЦЮТЖШдк35ЁцЁЃZn2+ГСЕэЭъШЋЃЌМДc(Zn2+)Ём10-5ЃЌc(Zn2+)=10-5ЪБЃЌ1.0ЁС1017=10-5ЁСc2(OH-)ЃЌНтЕУЃКc(OH-)=10-6ЃЌc(H+)=![]() =10-8ЃЌpH=8ЁЃЕБc(Zn2+)ЃМ10-5ЪБЃЌOH-ХЈЖШБШ10-6ДѓЃЌМюадИќЧПЃЌpHЃО8ЃЌЕБpH>11ЪБЃЌZn(OH) 2зЊЛЏЮЊ
=10-8ЃЌpH=8ЁЃЕБc(Zn2+)ЃМ10-5ЪБЃЌOH-ХЈЖШБШ10-6ДѓЃЌМюадИќЧПЃЌpHЃО8ЃЌЕБpH>11ЪБЃЌZn(OH) 2зЊЛЏЮЊ![]() ЃЌЫљвдЃЌЮЊСЫЪЙZn2+ГСЕэЭъШЋЃЌашПижЦpHЗЖЮЇдк[8-11)ЃЌЙЪД№АИЮЊЃКЮТЖШЕЭгк35ЁцЃЌЗДгІЫйТЪЬЋТ§ЃЌЮТЖШЬЋИпЃЌNa2S2O4ШнвзЗжНтЃЛ[8-11)ЃЛдіДѓc(Na+)ЃЌЪЙЦНКтNa2S2O4(s)
ЃЌЫљвдЃЌЮЊСЫЪЙZn2+ГСЕэЭъШЋЃЌашПижЦpHЗЖЮЇдк[8-11)ЃЌЙЪД№АИЮЊЃКЮТЖШЕЭгк35ЁцЃЌЗДгІЫйТЪЬЋТ§ЃЌЮТЖШЬЋИпЃЌNa2S2O4ШнвзЗжНтЃЛ[8-11)ЃЛдіДѓc(Na+)ЃЌЪЙЦНКтNa2S2O4(s)![]() 2Na+(aq)+ S2O42-(aq)ФцЯђвЦЖЏЃЌБугкNa2S2O4ОЇЬхЮіГіЃЛ
2Na+(aq)+ S2O42-(aq)ФцЯђвЦЖЏЃЌБугкNa2S2O4ОЇЬхЮіГіЃЛ
(4)ВНжшЂлжаМгШывЛЖЈСПNaClЙЬЬхЃЌc(Na+)діДѓЃЌЦНКтNa2S2O4(s)![]() 2Na+(aq)+ S2O42-(aq)ФцЯђвЦЖЏЃЌБугкNa2S2O4ОЇЬхЮіГіЃЌЙЪД№АИЮЊЃКЪЙc(Na+)діДѓЃЌЦНКтNa2S2O4(s)
2Na+(aq)+ S2O42-(aq)ФцЯђвЦЖЏЃЌБугкNa2S2O4ОЇЬхЮіГіЃЌЙЪД№АИЮЊЃКЪЙc(Na+)діДѓЃЌЦНКтNa2S2O4(s)![]() 2Na+(aq)+ S2O42-(aq)ФцЯђвЦЖЏЃЌБугкNa2S2O4НсОЇЮіГіЃЛ
2Na+(aq)+ S2O42-(aq)ФцЯђвЦЖЏЃЌБугкNa2S2O4НсОЇЮіГіЃЛ
(5)ВНжшЂлЮіГіNa2S2O4ОЇЬхЃЌВНжшЂмЮЊЙ§ТЫЃЌЯДЕгЃЌNa2S2O4ФбШмгкввДМЃЌЙЪгУввДМЯДЕгЃЌЯДЕгвЛАужиИД2-3ДЮЃЌМДВНжшЂмЮЊЙ§ТЫЃЌгУввДМЯДЕг2-3ДЮЃЌЙЪД№АИЮЊЃКЙ§ТЫЃЌгУввДМЯДЕг2-3ДЮЃЛ
(6)НсКЯЕЮЖЈЙ§ГЬЗЂЩњЕФЗДгІЗНГЬЪНПЩЕУЃКNa2S2O42H2O~NaHSO2CH2O~I2ЃЌЫљвдЃК ЃЌНтЕУЃКn=
ЃЌНтЕУЃКn=![]() ЃЌдђmgбљЦЗжаNa2S2O4ЕФЮяжЪЕФСП=
ЃЌдђmgбљЦЗжаNa2S2O4ЕФЮяжЪЕФСП=![]() =
=![]() ЃЌNa2S2O4ЕФжЪСП=210g/molЁС
ЃЌNa2S2O4ЕФжЪСП=210g/molЁС![]() =105cVЁС10-2gЃЌNa2S2O42H2OЕФДПЖШ=
=105cVЁС10-2gЃЌNa2S2O42H2OЕФДПЖШ=![]() =
=![]() ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК![]() ЁЃ
ЁЃ