��Ŀ����
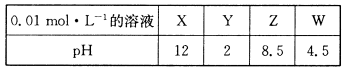
����25 ��ʱ0.1 mol��L��1�İ�ˮ����ش��������⣺
��1������ˮ�м�����������粒��壬һˮ�ϰ��ĵ���ƽ��________(����������ҡ�����)�ƶ�����ʱ��Һ�� ________(���������С�����䡱)��
________(���������С�����䡱)��
��2������ˮ�м����Ũ��ϡ���ᣬʹ��ǡ���кͣ�д����Ӧ�����ӷ���ʽ��_________________��������Һ��pH________7(���������<������)��
��3������ˮ�м���ϡ��������Һ��pH��7����ʱ[NH4+]��a mol��L��1����c(SO42-)��________��
��4������ˮ�м���pH��1�����ᣬ�Ұ�ˮ������������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����________________________________________��
��1������ ��С ��2��NH3��H2O��CH3COOH��CH3COO����NH4����H2O �� ��3��

��4��c(NH4��)��c(SO42��)��c(H��)��c(OH��)
���������������1����ˮ�м�����������粒��壬����淋��������笠����ӻ�����һˮ�ϰ��ĵ��룬ƽ�������ƶ�����ʱ��Һ�� ��С ��2��NH3��H2O��CH3COOH��CH3COO����NH4����H2O ����Ϊǡ���кͣ���Һ�����ԣ�������Һ��pH��7 ��3�����ݵ���غ��У�c(NH4��)��c(H��)��2c(SO42��)��c(OH��)������Ϊ��Һ��pH��7������У�c(SO42��)��
��С ��2��NH3��H2O��CH3COOH��CH3COO����NH4����H2O ����Ϊǡ���кͣ���Һ�����ԣ�������Һ��pH��7 ��3�����ݵ���غ��У�c(NH4��)��c(H��)��2c(SO42��)��c(OH��)������Ϊ��Һ��pH��7������У�c(SO42��)�� c(NH4��)��
c(NH4��)�� ����4������ˮ�м���pH��1�����ᣬ�Ұ�ˮ������������Ϊ1��1����õ���������淋���Һ����Ϊ�������ӵ�ˮ�⣬ʹ����Һ�����ԣ���������¹�ϵ��c(NH4��)��c(SO42��)��c(H��)��c(OH��)
����4������ˮ�м���pH��1�����ᣬ�Ұ�ˮ������������Ϊ1��1����õ���������淋���Һ����Ϊ�������ӵ�ˮ�⣬ʹ����Һ�����ԣ���������¹�ϵ��c(NH4��)��c(SO42��)��c(H��)��c(OH��)
���㣺����������ʵķ�Ӧ��

������ǿ�ᣬ��ѧ�ν�������ˮ��Һ�п�����ȫ���롣����ʵ�ǣ�������ˮ�еĵ�һ����������ȫ�ģ��ڶ������벢����ȫ����������ΪH2SO4=H����HSO4-��HSO4- H����SO42-��
H����SO42-��
��ش������й����⣺
��1��Na2SO4��Һ��________(������ԡ��������ԡ��������ԡ�)����������__________________________________________��(�����ӷ���ʽ��ʾ)��
��2��H2SO4��Һ��BaCl2��Һ��Ӧ�����ӷ���ʽΪ_________________________��
��3����0.10 mol��L��1��Na2SO4��Һ�У���������Ũ�ȵĹ�ϵ��ȷ����________(��д���)��
| A��c(Na��)��c(SO42-)��c(HSO4-)��c(H2SO4) |
| B��c(OH��)��c(HSO4-)��c(H��) |
| C��c(Na��)��c(H��)��c(OH��)��c(HSO4-)��2c(SO42-) |
| D��c(Na��)��2c(SO42-)��2c(HSO4-) |
��5����25��ʱ��0.10 mol��L��1H2SO4��Һ��pH����lg 0.11����0.10 mol��L��1H2SO4��Һ��c(SO42-)��________ mol��L��1��
ijѧ��������֪���ʵ���Ũ�ȵĴ������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���ʵ���ָʾ��������д���пհף�
��1���ñ�����ζ����������������Һʱ��������ѡ����ѡ����ǡ����һ��________��
| | ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� |
| A | �� | �� | ʯ�� | ���ң� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ��̪ | ���ף� |
| D | �� | �� | ʯ�� | ���ң� |
�ζ�ʱӦ��������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע�� ��ֱ������һ�δ������Һ��ɫ�� ɫ��Ϊ ɫ�����ڰ��������Һ��ɫ���ı�Ϊֹ��
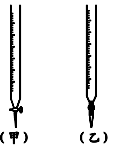
��2�����в����п���ʹ��������������Һ��Ũ��ֵƫ�͵��� ��
A.��ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B.�ζ�ǰʢ������������Һ����ƿ������ˮϴ����δ����
C.��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D.��ȡ�������ʱ����ʼ���Ӷ������ζ��������Ӷ���
��3��ijѧ������3��ʵ��ֱ��¼�й����������
| | ������������ | 0.100mol/L�������� | |
| �ζ����� | ��Һ�������ml�� | �ζ�ǰ�Ŀ̶ȣ�ml�� | �ζ���Ŀ̶ȣ�ml�� |
| ��һ�� | 25.00 | 0.00 | 24.98 |
| �ڶ��� | 25.00 | 1.56 | 27.86 |
| ������ | 25.00 | 0.22 | 25.24 |
�����ϱ����ݼ��������������Һ�����ʵ���Ũ��Ϊ ��
��4����ͼΪ����25 mL NaOH��Һ����εμ�CH3COOH��Һ��������ҺpH�ı仯���ߣ���ش�
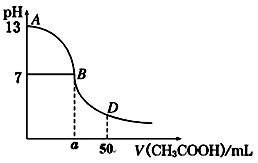
��B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��________��ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�________���䣮������ȷ�����ʲ��𣩡�
����D��ʱ����Һ��c��CH3COO������c��CH3COOH��________2c��Na���������������������������
��1����������pH=12��NaCN��Һ�У���ˮ�����c(OH��)Ϊ mol?L��1��
��2��Ũ��Ϊ0.1mol?L��1�����и����ʵ���Һ�У�c(NH4+)�ɴ�С��˳����___������ţ���
��NH4Cl ��NH4HSO4 ��NH3?H2O ��CH3COONH4
��3��ij��Ԫ�ᣨ��ѧʽ��H2A��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�
H2A=H+ +HA����HA�� H+ +A2����
H+ +A2����
����Na2A��Һ��____�ԣ�NaHA��Һ�� �ԣ�����ԡ��������ԡ����ԡ�����
������0.1mo1?L��1Na2A����Һ�����и�������Ũ���ɴ�С��˳���ǣ� ������ţ���
| A��c(Na+)>c(A2��)>c(OH��)>c(HA��)>c(H+) |
| B��c(Na+)> c(OH��)>c(HA��)> >c(A2��) > c(H+) |
| C��c(Na+)> c(H+)> c(A2��)> c(OH��)>c(HA��) |
| D��c(A2��)>c(Na+)> c(OH��) > c(H+)>c(HA��) |
�ٵ�AgBr������ʼ����ʱ����Һ��Ag+Ũ���� ��
�ڵ�AgC1������ʼ����ʱ����Һ�е�Br����I���Ƿ���ȫ���� ������Һ������Ũ��С��1.0��10��5mo1/Lʱ����Ϊ�Ѿ�������ȫ������ѡ��ǡ�����
ijѧϰС����̽��CaSO4����ת��ΪCaCO3�������Ӷ������ȥ�Ŀ����ԣ�����������ϣ�(�������ݺ�ʵ���ָ��25���²ⶨ)
| ���ܵ���� | CaCO3 | CaSO4 |
| Ksp(mol2��L��2) | 3��10��9 | 9��10��6 |
ʵ�鲽�����£�
����100 mL 0.1 mol��L��1��CaCl2��Һ�м���100 mL 0.1 mol��L��1��Na2SO4��Һ�������а�ɫ�������ɡ�
������������Һ�м������Na2CO3 3 g�����裬���ã���������ȥ�ϲ���Һ��
���ټ�������ˮ���裬���ã�����������ȥ�ϲ���Һ��
��������м������������ᡣ
��1���������������Һ��[Ca2��]��________ mol��L��1
��2��д���ڢڲ�������Ӧ�����ӷ���ʽ��________________________________.
��3����Ƶڢ۲���Ŀ����________________________________________________��
��4���ڢܲ���������������Ϊ�� ��