��Ŀ����
����Ŀ����������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na+��NH4+��Mg2+��Al3+��SO42����NO3����Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ������������Һ����Ʋ���������µ�ʵ�飺

��֪��3NO3��+ 8Al + 5OH�� + 2H2O![]() 3NH3��+ 8AlO2��
3NH3��+ 8AlO2��
�������ϵ�ʵ�����������ͬѧ�ó��Ľ�������ȷ����
A�������п϶�����NH4+��Mg2+��SO42����NO3��
B�������п��ܴ���Na+��Cl��
C��������һ������Al3+
D���������п��ܴ���NaNO3��NH4Cl��MgSO4
���𰸡�C
��������
������������������������������������ʪ���ɫʯ����ֽ������˵����笠�������1���������ӣ����������ܽ�˵������þ���Ӻ��������ͨ�������̼���ɳ���2���������ӳ����ܽⲢ�ų����壬˵��������̼�ᱵ������Ҳ������������������������3NO3��+ 8Al + 5OH�� + 2H2O![]() 3NH3��+ 8AlO2����˵����Һ��һ�������������A��ȷ����Һ�п��ܺ��������ӣ����������������������Ӧ����ƫ�������ͨ������̼��������������������2�У������������ܽ⣬C���������п��ܴ���Na+��Cl����B��ȷ���������п��ܴ���NaNO3��NH4Cl��MgSO4��D��ȷ����ѡC��
3NH3��+ 8AlO2����˵����Һ��һ�������������A��ȷ����Һ�п��ܺ��������ӣ����������������������Ӧ����ƫ�������ͨ������̼��������������������2�У������������ܽ⣬C���������п��ܴ���Na+��Cl����B��ȷ���������п��ܴ���NaNO3��NH4Cl��MgSO4��D��ȷ����ѡC��

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�����Ŀ��
����ʳ�γ���������Ca2+��Mg2+��SO42-���������ӣ�ʵ�����ᴿ NaCl ���������£�
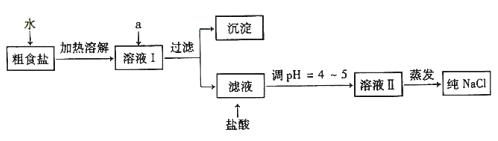
�ṩ���Լ���Na2CO3��Һ��K2CO3��Һ��NaOH ��Һ��Ca��OH��2��Һ��BaCl2��Һ��Ba��NO3��2��Һ��ϡ���ᡢϡ���ᡢAgNO3��Һ��
������ȥ��Һ���е� Ca2+��Mg2+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ_________���ѧʽ����
������֮ǰ����������SO42-�ѳ�ȥ��___________��
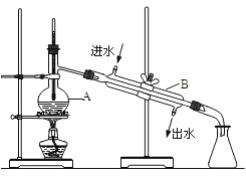
������Ҫ480mL1.00mol��L-1NaCl��Һ��
��������������Һ�������ò��������г����˲����漰�����⣬����___________��
���������� NaCl������Ϊ___________��
�����в�������ȷ˳���ǣ�����ĸ��ʾ�� B��_______��______��_______��_______��_______��G ��
A���µߵ�ҡ�� B���� Cϴ�� D���� E�ܽ� F��Һ Gװƿ
�����в�����������ҺŨ���к�Ӱ�죬�ں�������д��ƫ��������ƫ����������Ӱ������
A������ʱ�������⣺__________�� | B���ܽ�ǰ�ձ�����ˮ��__________�� |
C������ƿϴ�Ӻ�δ����___________�� | D������ʱ��������ƿ�̶��ߣ�___________�� |