ƒøƒ⁄»ð
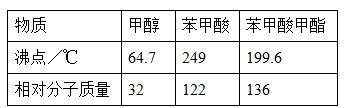
(11∑÷) °∞æ∆ «≥¬µƒœ„°±£¨æÕ «“ÚŒ™æ∆‘⁄¥¢¥Êπ˝≥Ã÷–…˙≥…¡À”–œ„Œ∂µƒ““À·““ı•£¨‘⁄ µ—È “Œ“√«“≤ø…“‘”√»ÁÕºÀ˘ 浃◊∞÷√÷∆»°““À·““ı•°£ªÿ¥œ¬¡–Œ £∫
£®1£©–¥≥ˆ÷∆»°““À·““ı•µƒªØ—ß∑¥”¶∑Ω≥Ã Ω£∫
°°°°°°°°°°°°°°°°°°°°°°°°
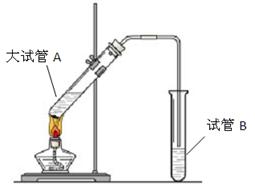
£®2£© ‘πÐB÷– ¢∑≈µƒ»Ð“∫ « £¨∆‰÷˜“™◊˜”√ «
°£
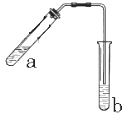
£®3£©◊∞÷√÷–Õ®’Ù∆¯µƒµºπÐ≤ªƒÐ≤»Π‘πÐBµƒ»Ð“∫÷–£¨ƒøµƒ «°°°°°°°°°°°°°°°°°°°£
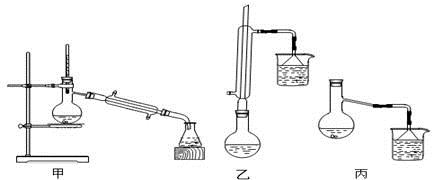
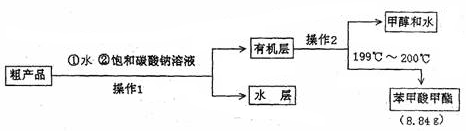
£®4£©»Ù“™∞—÷∆µ√µƒ““À·““ı•∑÷¿Î≥ˆ¿¥£¨”¶≤…”√µƒ µ—È≤Ÿ◊˜ «°°°°°°°°°°°°°°°£
£®5£©◊ˆ¥À µ—È ±£¨Õ˘Õ˘ªπœÚ¥Û ‘πÐA÷–º”»Îº∏øÈÀÈ¥…∆¨£¨∆‰ƒøµƒ «°°°°°°°°°°°°°£
£®6£©”√30g““À·”Î46g““¥º∑¥”¶£¨»Áπ˚ µº ≤˙¡ø «¿Ì¬€≤˙¡øµƒ67%£¨‘Ú µº µ√µΩ““À·““ı•µƒ÷ ¡ø «°°°°°°°°°°°°°£

£®1£©–¥≥ˆ÷∆»°““À·““ı•µƒªØ—ß∑¥”¶∑Ω≥Ã Ω£∫
°°°°°°°°°°°°°°°°°°°°°°°°
£®2£© ‘πÐB÷– ¢∑≈µƒ»Ð“∫ « £¨∆‰÷˜“™◊˜”√ «
°£
£®3£©◊∞÷√÷–Õ®’Ù∆¯µƒµºπÐ≤ªƒÐ≤»Π‘πÐBµƒ»Ð“∫÷–£¨ƒøµƒ «°°°°°°°°°°°°°°°°°°°£
£®4£©»Ù“™∞—÷∆µ√µƒ““À·““ı•∑÷¿Î≥ˆ¿¥£¨”¶≤…”√µƒ µ—È≤Ÿ◊˜ «°°°°°°°°°°°°°°°£
£®5£©◊ˆ¥À µ—È ±£¨Õ˘Õ˘ªπœÚ¥Û ‘πÐA÷–º”»Îº∏øÈÀÈ¥…∆¨£¨∆‰ƒøµƒ «°°°°°°°°°°°°°£
£®6£©”√30g““À·”Î46g““¥º∑¥”¶£¨»Áπ˚ µº ≤˙¡ø «¿Ì¬€≤˙¡øµƒ67%£¨‘Ú µº µ√µΩ““À·““ı•µƒ÷ ¡ø «°°°°°°°°°°°°°£
| A£Æ44g | B£Æ29.5g | C£Æ74.8g | D£Æ88g |
£®1£©CH3COOH +C2H5OH CH3COOC2H5+H2O £®2∑÷£©
CH3COOC2H5+H2O £®2∑÷£©
£®2£©±•∫ÕNa2CO3»Ð“∫£ªΩµµÕı•‘⁄ÀÆ÷–µƒ»ÐΩ‚∂»£¨≥˝»•ı•÷–ªÏ”–µƒ¥º∫ÕÀ·£¨¿˚”⁄∑÷≤„°££®1+3∑÷£©
£®3£©µπŒ¸£®1∑÷£© £®4£©∑÷“∫£®1∑÷£© £®5£©∑¿÷π±©∑–£®1∑÷£©£®6£©B£®2∑÷£©
 CH3COOC2H5+H2O £®2∑÷£©
CH3COOC2H5+H2O £®2∑÷£©£®2£©±•∫ÕNa2CO3»Ð“∫£ªΩµµÕı•‘⁄ÀÆ÷–µƒ»ÐΩ‚∂»£¨≥˝»•ı•÷–ªÏ”–µƒ¥º∫ÕÀ·£¨¿˚”⁄∑÷≤„°££®1+3∑÷£©
£®3£©µπŒ¸£®1∑÷£© £®4£©∑÷“∫£®1∑÷£© £®5£©∑¿÷π±©∑–£®1∑÷£©£®6£©B£®2∑÷£©
£®1£© µ—È “÷∆»°““À·““ı•”√““À·∫Õ““¥ºÕ®π˝ı•ªØ∑¥”¶…˙≥…£¨∑Ω≥à ΩCH3COOH +C2H5OH CH3COOC2H5+H2O°£
CH3COOC2H5+H2O°£
£®2£©‘⁄∑¥”¶π˝≥Ã÷–£¨““¥º∫Õ““À·∂º““ª”∑¢£¨À˘“‘“™≥˝»•““À·““ı•÷–µƒ““À·∫Õ““¥º£¨ø…“‘¿˚”√±•∫ÕúÀ·ƒ∆»Ð“∫£¨∆‰◊˜”√ «ΩµµÕı•‘⁄ÀÆ÷–µƒ»ÐΩ‚∂»£¨≥˝»•ı•÷–ªÏ”–µƒ¥º∫ÕÀ·£¨¿˚”⁄∑÷≤„∫ÕŒ≈µΩ∆¯Œ∂°£
£®3£©”…”⁄““À·∫Õ““¥º∂º «∫ÕÀƪ•»Ðµƒ£¨À˘“‘Œ™¡À∑¿÷πµπŒ¸£¨µºπÐ≤ªƒÐ≤»λГ∫÷–°£
£®4£©““À·““ı•≤ª»Ð”⁄ÀÆ£¨∑÷“∫º¥ø…°£
£®5£©‘⁄∑¥”¶π˝≥Ã÷–£¨–Ë“™º”»»£¨“Ú¥Àº”»ÎÀÈ¥…∆¨µƒƒøµƒ «∑¿÷π±©∑–°£
£®6£©∏˘æð∑Ω≥à Ωø…÷™£¨46g““¥º»Áπ˚ÕÍ»´ı•ªØ–Ë“™60g““À·£¨“Ú¥À““À· «≤ª◊„µƒ£¨À˘“‘”¶∏√…˙≥…µƒ““À·““ı• «44g£¨À˘“‘ µº …˙≥…µƒ““À·““ı• «44g°¡67£•£Ω29.48g£¨¥∞∏—°B°£
 CH3COOC2H5+H2O°£
CH3COOC2H5+H2O°££®2£©‘⁄∑¥”¶π˝≥Ã÷–£¨““¥º∫Õ““À·∂º““ª”∑¢£¨À˘“‘“™≥˝»•““À·““ı•÷–µƒ““À·∫Õ““¥º£¨ø…“‘¿˚”√±•∫ÕúÀ·ƒ∆»Ð“∫£¨∆‰◊˜”√ «ΩµµÕı•‘⁄ÀÆ÷–µƒ»ÐΩ‚∂»£¨≥˝»•ı•÷–ªÏ”–µƒ¥º∫ÕÀ·£¨¿˚”⁄∑÷≤„∫ÕŒ≈µΩ∆¯Œ∂°£
£®3£©”…”⁄““À·∫Õ““¥º∂º «∫ÕÀƪ•»Ðµƒ£¨À˘“‘Œ™¡À∑¿÷πµπŒ¸£¨µºπÐ≤ªƒÐ≤»λГ∫÷–°£
£®4£©““À·““ı•≤ª»Ð”⁄ÀÆ£¨∑÷“∫º¥ø…°£
£®5£©‘⁄∑¥”¶π˝≥Ã÷–£¨–Ë“™º”»»£¨“Ú¥Àº”»ÎÀÈ¥…∆¨µƒƒøµƒ «∑¿÷π±©∑–°£
£®6£©∏˘æð∑Ω≥à Ωø…÷™£¨46g““¥º»Áπ˚ÕÍ»´ı•ªØ–Ë“™60g““À·£¨“Ú¥À““À· «≤ª◊„µƒ£¨À˘“‘”¶∏√…˙≥…µƒ““À·““ı• «44g£¨À˘“‘ µº …˙≥…µƒ““À·““ı• «44g°¡67£•£Ω29.48g£¨¥∞∏—°B°£

¡∑œ∞≤·œµ¡–¥∞∏
œýπÿƒø