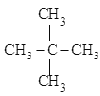
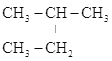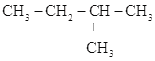��Ŀ����
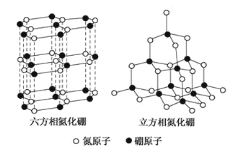
����Ŀ��������BN�������ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ����в�״�ṹ�������������������ܵ��硣�����൪�����dz�Ӳ���ϣ����������ĥ�ԡ����ǵľ���ṹ��ͼ��ʾ�����������־����˵������ȷ����
A. �����൪������λ��B��N
B. �����൪������������С�������ʵ������۵��
C. ���ֵ������е���ԭ�Ӷ��Dz���sp2�ӻ�
D. �����൪��������ṹ��ʯī����ȴ�����磬ԭ����û�п��������ƶ��ĵ���
���𰸡�D
��������
A��Bԭ���������3�����ӣ�����һ���չ����Nԭ���������5�����ӣ�����һ���µ��Ӷԣ��ɾ���ṹ��֪�������൪����Ϊ�ռ���״�ṹ����ʯ���ƣ������൪������B�γ�4�����ۼ�������1��ΪB��N��λ������A����
B�������൪������в�״�ṹ�����������С�������ʵ�����������ԭ�Ӽ�ͨ�����ۼ���ϣ������۵�ܸߣ���B����
C�������൪��������ԭ���γ�4�����ۼ������������൪��������ԭ�Ӳ��õ���sp3�ӻ�����C����
D�������д��ڿ��������ƶ��ĵ����ܵ��磬�����൪��������û�п��������ƶ��ĵ��ӣ����Բ����磬��D��ȷ��
��ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ҵ��ˮ�н������е������е�5�֣�������ˮ�ĵ��뼰���ӵ�ˮ�⣩���Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1mol/L��
������ | K+ Cu2+ Fe3+ Al3+ Fe2+ |
������ | Cl�� CO32- NO3�� SO42�� SiO32�� |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
I.�ò�˿պȡ������Һ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��
��.ȡ������Һ������KSCN��Һ�����Ա仯��
��.��ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䡣
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)���м�����������������ɫ��������ӷ���ʽ��____________________��
(2)�����к���ɫ���壨����£��ռ�һ���Թ�Ȼ����ˮ�У��������ʲ���ɢ����������Һ�����ʵ���Ũ��Ϊ___________mol/L����ȷ��ǧ��λ����
(3)��ͬѧ����ȷ��ԭ��Һ������������__________________��
(4)��ȡ100mLԭ��Һ������������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ��õ��Ĺ�������Ϊ_____________g��
����Ŀ���������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ��������������鼰���ǵĻ�����㷺Ӧ���ڳ�������ϵ�������ش��������⣺
��1��Fe2+�ĺ�������Ų�ʽΪ_________________��
��2��NH3��һ�ֺܺõ����壬NH3�ķе�______(����>����=������<��)AsH3��
��3��Nԭ�Ӻ�����______�ֲ�ͬ�˶�״̬�ĵ��ӡ���̬Nԭ���У�������ߵĵ�����ռ�ݵ�ԭ�ӹ������״Ϊ_____��
��4����ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ��ͼ��ͼ�����߱�ʾ��������Ϊ________��
��5��As��±������۵����£�
AsCl3 | AsBr3 | AsI3 | |
�۵�/K | 256.8 | 304 | 413 |
����±�����۵�����ԭ����________________��
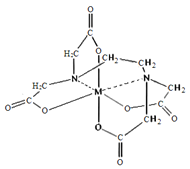
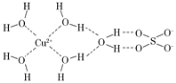
��6����FeCl3��Һ�е���EDTA�Լ��ɵ������A,��ṹ��ͼ��ʾ��ͼ��M����Fe3+����Fe3+�뵪ԭ��֮���γɵĻ�ѧ����_______��Fe3+����λ��Ϊ______��