��Ŀ����
����Ŀ��ijѧϰС������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ��������
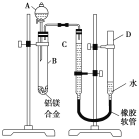
(1)A���Լ�Ϊ_________________��
(2)ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����__________________________��
(3)��������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�еμ������Լ�������������˳����__________________ (�����)����¼C��Һ��λ��ʱ����ƽ���⣬��Ӧ_________________��
(4)B�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
(5)��ʵ������þ�Ͻ������Ϊa g������������Ϊb mL(�ѻ���Ϊ��״��)��B��ʣ����������Ϊc g�����������ԭ������Ϊ_____________________��
���𰸡�NaOH��Һ ��ȥ��þ�Ͻ���������Ĥ �٢ܢۢ� ʹD��C��Һ����ƽ 2Al+2NaOH+2H2O��2NaAlO2+3H2�� ![]()
��������
������ǿ�Ӧ��þ���ܣ�������һ�������ʿɴﵽʵ��Ŀ�ġ��ⶨ�Ͻ����������������ɳ�ȡһ�����Ͻ���Ʒ������������������������������ԭ���������ɲ���Ħ������=��������/�������ʵ�������������������������������ʵ�����
(1)A���Լ�Ϊǿ����Һ��������NaOH��Һ�ȡ�
(2)����þ���ǻ��ý������ڿ����б����γ���������Ĥ��ʵ��ǰ������þ�Ͻ���ϡ���н���Ƭ�̣�����Ҫ��ȥ���������Ĥ��
(3)ʵ������Ҫ�����Ʒ��������������Ӧ����������������ڼ�������ԡ�ҩƷ��ˮװ�����������Ӻ�װ�ú�����¼C��Һ��λ�ã�����A��B�еμ������Լ�������B�в���������������ָ������º�¼C��Һ��λ�ã�����B��ʣ�������ˣ�ϴ�ӣ�������أ�������˳�����٢ܢۢ�����¼C��Һ��λ��ʱ��Ӧ����D�ܸ߶�ʹD��C��Һ����ƽ����ƽ�Ӷ�����
(4)�Թ�B�ڣ��Ͻ��е�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O��2NaAlO2+3H2����
(5)��Al�����ԭ������Ϊx����
2Al ~ 3H2
2x g 3��22.4 L
(a-c) g b��10-3 L
��x=![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��N2(g)+3H2(g)![]() 2NH3(g) ��H��0���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±�������������⡣
2NH3(g) ��H��0���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±�������������⡣
t/�� | 25 | 125 | 225 | �� |
K | 4.1��105 | K1 | K2 | �� |
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=________��K1______K2����������������������������������ѹǿʹƽ��������Ӧ�����ƶ�����ƽ�ⳣ��_________������������������������
��2���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������____________������ţ���
A��2��H2������= 3��NH3���棩 B�����������ܶȱ��ֲ���
C��������ѹǿ���ֲ��� D��N2���������ʵ���H2����������
E�������������ƽ����Է�����������ʱ����仯 F������������ɫ���ֲ���
��3������ͬ����N2��H2�ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У�����������Ӧ�õ������������ݣ�
ʵ���� | �¶ȣ��棩 | ��ʼ����mol�� | ƽ������mol�� | �ﵽƽ������ʱ�䣨min�� | |
N2 | H2 | NH3 | |||
1 | 650 | 2 | 4 | 0.9 | 9 |
2 | 900 | 1 | 2 | 0.3 | 0.01 |
ʵ��1������(NH3)��ʾ�ķ�Ӧ����Ϊ______��ʵ��2�����ʱ�ʵ��1���ԭ����____________��
��4��������Ʋ��ø��������ӵ������SCY�մɣ��ܴ���H+����ʵ���˳�ѹ�¼��ܺϳɰ����ܷ����ʵ��װ�ã���ͼ�������������ĵ缫��ӦΪ_____________��

����Ŀ���������������ܰ뵼�����Ҫԭ�ϡ���ҵ�ϳ���п��ұ���ķ����л����ء���֪ijп������Ҫ��Zn��Si��Pb��Fe��Ga����������øÿ������صĹ����������£�
��֪��������Ԫ�����ڱ���λ�ڵ������ڵڢ�A�壬��ѧ�����������ơ�
��lg2=0.3 lg3=0.48��
�۲������ʵ�Ksp���±���ʾ��
���� | Zn(OH)2 | Ga(OH)3 | Fe(OH)2 | Fe(OH)3 |
Ksp | 1.6��10��17 | 2.7��10��31 | 8��10��16 | 2.8��10��39 |
(1)Ϊ�����������ʣ����ʵ���������Ũ���⣬Ӧ��ȡ�Ĵ�ʩ��__________________��д��������������1����Ҫ�ɷ�������Ǧ��_________________(д��ѧʽ)��
(2)����H2O2��Ŀ����(�����ӷ���ʽ��ʾ)______________________ ��
(3)���������£�������Һ�и������ӵ�Ũ�Ⱦ�Ϊ0.01mo/L������Һ��ij������Ũ��С��1��10-5mol/Lʱ����Ϊ����������ȫ��ȥ����pHӦ���ڵķ�ΧΪ___________________��
(4)����D������_______________���ˡ�ϴ�ӡ����
(5)��ⷨ�Ʊ������ء��ö��Ե缫���NaGaO2��Һ�����Ƶý����أ�д�������缫��Ӧʽ________________________��