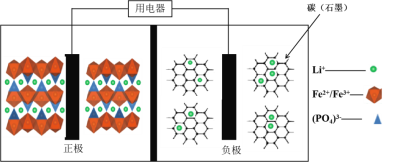
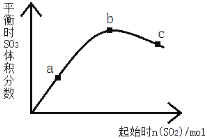
��Ŀ����
����Ŀ����1�������£���10mL0.05mol/LH2SO4��Һ��ˮϡ����100mL��ϡ�ͺ���Һ��pH=_____
��2��ij�¶�ʱ�����0.01mol��L��1��NaOH��ҺpH��10������¶���ˮ�����ӻ�����KW��____���ڸ��¶��£���pH��10��NaOH��ҺaL��pH��1��ϡ����bL��ϣ������Ϻ���Һ�����С�仯���Բ��ƣ��������û��ҺpH��2����a��b��____��
��3����6gCH3COOH����ˮ�Ƴ�1L��Һ�����ⶨ��Һ�к�CH3COO�CΪ1.4��10�C3mol/L�����¶��´���ĵ��볣����Ka��_____
���𰸡�2 1��10��12 9��2 1.96��10-5
��������
(1)10mL0.05mol/L��H2SO4��Һ��ˮϡ�͵�100mL��c(H+)��0.1mol/L��Ϊ0.01mol/L��
(2)�����£�0.01molL-1��NaOH��Һ��pHΪ12����ij�¶�ʱ���0.01molL-1��NaOH��Һ��pHΪ10����KW���������û����Һ��pH=2�����������ʽ�ҳ�c(H+)���������ʵ����Ĺ�ϵ���ټ����a��b��
(3)��6gCH3COOH����ˮ�Ƴ�1L��Һ�����ⶨ��Һ�к�CH3COO-Ϊ1.4��10-3mol/L���������̶Ƚ�С������Һ�д���c(H+)��c(CH3COO-)=1.4��10-3mol/L��c(CH3COOH)=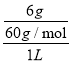 =0.1mol/L�����¶��´���ĵ��볣����Ka=
=0.1mol/L�����¶��´���ĵ��볣����Ka=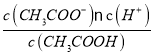 ��
��
(1)10mL0.05mol/L��H2SO4��Һ��ˮϡ�͵�100mL��c(H+)��0.1mol/L��Ϊ0.01mol/L����ϡ�ͺ���Һ��pH=-lg0.01=2��
(2)�����£�0.01molL-1��NaOH��Һ��pHΪ12����ij�¶�ʱ���0.01molL-1��NaOH��Һ��pHΪ10����KW=0.01��10-10=1.0��10-12�������û����Һ��pH=2�����Һ������������¶���pH��10��NaOH��Һ��c(OH-)=0.01molL-1��pH=1��ϡ������Һ��c(H+)=0.1 molL-1�����Ϻ���Һ��c(H+)=![]() =0.01 molL-1����ã�a:b=9:2��
=0.01 molL-1����ã�a:b=9:2��
(3)��6gCH3COOH����ˮ�Ƴ�1L��Һ�����ⶨ��Һ�к�CH3COO-Ϊ1.4��10-3mol/L���������̶Ƚ�С������Һ�д���c(H+)��c(CH3COO-)=1.4��10-3mol/L��c(CH3COOH)=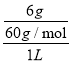 =0.1mol/L�����¶��´���ĵ��볣����Ka=
=0.1mol/L�����¶��´���ĵ��볣����Ka=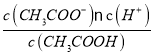 =
=![]() =1.96��10-5��
=1.96��10-5��

����Ŀ��ʵ������һδ֪Ũ�ȵ����ᣬijͬѧ��ʵ�����н���ʵ��ⶨ�����Ũ�ȡ������������գ�
����100mL0.10molL-1NaOH����Һ������������ƽ����___g���������ƹ��塣
ȡ20.00mL�������������ƿ�У����μ� 2~3�η�̪��ָʾ���������Ƶı� NaOH��Һ���еζ����ظ������ζ����� 2~3�Σ���¼�������£�
�ζ����� | ������������/mL | 0.10 mol/L NaOH ��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
��һ�� | 20.00 | 2.00 | 28.15 |
�ڶ��� | 20.00 | 1.50 | 29.50 |
������ | 20.00 | 0.20 | 26.55 |
�ٸ�ʵ��ζ��ﵽ�յ�ı�־��_________
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ______����������λ��Ч���֣�
��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ���_______����˫ѡ��
A �ζ��յ����ʱ���Ӷ��� B ��ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
C ��ƿˮϴ��δ���� D ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
E ����NaOH ����ʱ����������KOH����
����������ʵ�����Ƶ�0.10molL��1NaOH��Һ�ζ�δ֪Ũ�ȵ�CHCOOH��Һ����Ӧǡ����ȫʱ��������������ȷ����_____
A ��Һ�����ԣ���ѡ�ü��Ȼ��̪��ָʾ��
B ��Һ�����ԣ�ֻ��ѡ��ʯ����ָʾ��
C ��Һ�ʼ��ԣ���ѡ�ü��Ȼ��̪��ָʾ��
D ��Һ�ʼ��ԣ�ֻ��ѡ�÷�̪��ָʾ��