��Ŀ����
10�������A��Ԫ�ط������������ٷ����ΪCr��19.5%��Cl��40.0%��H��4.50%��O��36%����0.533gA����100mLˮ�У��ټ�������HNO3ʹ���ܽ⣬Ȼ��������AgNO2��Һ��������ȫ�������������ﴦ��������0.287g����֪1.06gA�ڸ�������л���������100��ʱ��0.144gˮ�ͷţ���ش��������⣺��1�������A��ʵ��ʽ��CrCl3H12O6
��2�������A�Ļ�ѧʽ��[CrCl2��H2O��4]Cl•2H2O
��3�������A�ļ����칹����2�֣�
���� ��1�����������A�ĸ�Ԫ�������������������A��Cr��Cl��H��Oԭ����Ŀ�ȣ���������A��ʵ��ʽ��
��2������������������ˮ��Һ���ܷ������룬�Ƚ�ԭ�Ӳ��ܷ������룬���������λ����Cl-����Ag+��Ӧ���������Cl-������Ag+��Ӧ�������Ȼ�����������������A��������ӵ����ʵ���������1.06gA�ڸ�������л���������100��ʱ��0.144gˮ�ͷţ��������������ˮ�����ʵ��������������⻯ѧʽ��
��3�������A�ļ����칹����˳���칹���֣�
��� �⣺��1�������A�У�Cr��19.5%��Cl��40.0%��H��4.50%��O��36%����N��Cr����N��Cl����N��H����N��O��=$\frac{19.5}{52}$��$\frac{40}{35.5}$��$\frac{4.5}{1}$��$\frac{36}{16}$=1��3��12��6�����������A��ʵ��ʽΪ��CrCl3H12O6��
�ʴ�Ϊ��CrCl3H12O6��
��2��0.533gA��Cl��40.0%��n��Cl-��=$\frac{0.533g��40%}{35.5g/mol}$=0.006mol����0.533gA����100mLˮ�У��������AgNO3��Һ��������ȫ�������������ﴦ��������0.287gΪ�Ȼ���������Ag++Cl-=AgCl����n��Cl-��=$\frac{0.287g}{143.5g/mol}$=0.002mol������������������ˮ��Һ���ܷ������룬�Ƚ�ԭ�Ӳ��ܷ������룬���������λ����Cl-����Ag+��Ӧ���������Cl-������Ag+��Ӧ������������Ӻ��ڽ������ӱ�Ϊ��0.002����0.006-0.002��=1��2��0.533gA��H��4.50%��n��H��=$\frac{0.533g��4.50%}{1g/mol}$=0.023985mol��n��H2O��=$\frac{1}{2}��$0.023985mol=0.0119925mol��1.06gA��n��H2O��=0.02385mol��1.06gA�ڸ�������л���������100��ʱ��0.144gˮ��n��H2O��=$\frac{0.144g}{18g/mol}$=0.008mol�����ڽ�ˮ���Ӻ����ˮ���ӱ�Ϊ����0.02385mol-0.008mol����0.008mol=2��1��
�������A��ʵ��ʽΪ��CrCl3H12O6�����������A�Ļ�ѧʽ��[CrCl2��H2O��4]Cl•2H2O��
�ʴ�Ϊ��[CrCl2��H2O��4]Cl•2H2O��
��3�����崦�����ڵ�λ��Ϊ˳ʽ��������Ե�λ��Ϊ��ʽ����[CrCl2��H2O��4]Cl•2H2O�ڽ����������Ӵ�������Ϊ˳ʽ���������Ϊ��ʽ�����������A�ļ����칹����˳���칹���֣�
�ʴ�Ϊ��2��
���� ���⿼���������A��ѧʽ�ļ��㣬��Ŀ�Ѷ��еȣ�ע���������ø�Ԫ�ص���������ȷ�������A�и�ԭ�ӵ���Ŀ�ȣ���ȷ���ʃȽ硢���������ǽ��2���Ĺؼ�������������ѧ��������û���֪ʶ��������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | SnH4��GeH4��SiH4��CH4 | B�� | SbH3��AsH3��PH3��NH3 | ||
| C�� | HI��HBr��HCl��HF | D�� | H2Te��H2Se��H2S��H2O |
| A�� | ����ɫ�Լ�ƿʢ��Ũ���� | B�� | ����������ʢ��Ũ���� | ||
| C�� | �ò����Լ�ƿʢ������� | D�� | �ô��������Լ�ƿʢ�ű� |
 ʵ�����в����������ȡ������ʵ��װ����ͼ��ʾ��ʡ�Լӳֺ;���װ�ã���c�������ж���ȷ���ǣ�������
ʵ�����в����������ȡ������ʵ��װ����ͼ��ʾ��ʡ�Լӳֺ;���װ�ã���c�������ж���ȷ���ǣ�������| ѡ�� | �Լ�a | �Լ�b | �Լ�c | c�е����� |
| A | Ũ��ˮ | ��ʯ�� | ��������Һ | �ȳ�������ʧ |
| B | ϡ���� | �� | ˮ | �Թܿ����������� |
| C | Ũ���� | ������� | ʯ����Һ | ��Һ��ɫû�б仯 |
| D | ϡ���� | ���� | ��������Һ | �ȳ�������ʧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
��ʽ̼���ܣ�Co4��OH��,��CO3��4�ݳ��������Ӳ��ϡ����Բ��ϵ����Ӽ���������ˮ������ʱ�ɷֽ��������������Ϊ��ȷ������ɣ�ij��ѧ��ȤС��ͬѧ�������ͼ��ʾ��װ�ã����������������顣
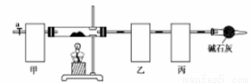
ʵ�鲽�����£�
�ٳ�ȡ3.65g��Ʒ����Ӳ�ʲ������ڣ������ҡ���װ�õ�������
�ڰ���ͼ��ʾװ����װ������������ ��
�ۼ���Ӳ�ʲ����ܣ�����װ���� ������ֹͣ���ȣ�
�ܴ���a������ͨ����������Ӻ����ҡ���װ�õ�������
�ݼ��㡣
��1��������ͼʾѡ��������װ�����ڷ����У�ʹ����ʵ��װ��������ѡ����ĸ��ţ����ظ�ѡ��
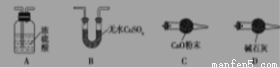
�ף� �ң� ����
��װ�õ������� ��
��2���������ʡ�Ե�ʵ�����Ϊ ��
���������װ�õ�����Ϊ ��
������л���ͨ����������ӵ�Ŀ���� ��
��3��������ȷװ�ý���ʵ�飬����������ݡ�
��װ�õ�����/g | ��װ�õ�����/g | |
����ǰ | 80.00 | 62.00 |
���Ⱥ� | 80.36 | 62.88 |
��ü�ʽ̼���ܵĻ�ѧʽΪ_____________��
��4��CO2��SO2��Ϊ�������壬�������ơ�Ϊ�˱Ƚ��������̼�������ǿ����ijͬѧ������װ�ý���ʵ�顣
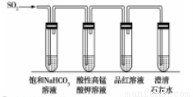
��д����ʵ���ܴﵽʵ��Ŀ�ĵ�ʵ������____________��
������SO2ͨ��ˮ�������ͣ������ʵ��֤�������������ᣬʵ�鷽��Ϊ____________��
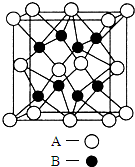 A��B��C��D��E��Ԫ�����ڱ���ԭ���������ε�����ǰ������Ԫ�أ�Aԭ������������Ϊ�ڲ��������3����B����ɫ��Ӧ�ʻ�ɫ��C���⻯����һ��ǿ�ᣬ��Ũ��Һ����A��E�Ļ����ﷴӦ����C�ĵ��ʣ�D��һ�ֽ���Ԫ�أ����̬ԭ������6��δ�ɶԵ��ӣ���ش��������⣺
A��B��C��D��E��Ԫ�����ڱ���ԭ���������ε�����ǰ������Ԫ�أ�Aԭ������������Ϊ�ڲ��������3����B����ɫ��Ӧ�ʻ�ɫ��C���⻯����һ��ǿ�ᣬ��Ũ��Һ����A��E�Ļ����ﷴӦ����C�ĵ��ʣ�D��һ�ֽ���Ԫ�أ����̬ԭ������6��δ�ɶԵ��ӣ���ش��������⣺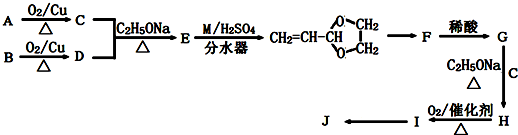
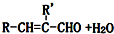

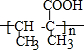 ��
��