��Ŀ����
16��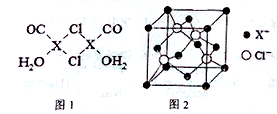 Ԫ��X�Ļ�̬ԭ���еĵ��ӹ���7���ܼ���������������Ϊ1��Xԭ�ӵ��ڲ���ȫ���������ӣ�����������У�����XCl��������Һ���ղ������ⶨCO�ĺ������仯ѧ��Ӧ���£�2XCl+2CO+2H2O�TX2Cl2•2CO•2H2O��
Ԫ��X�Ļ�̬ԭ���еĵ��ӹ���7���ܼ���������������Ϊ1��Xԭ�ӵ��ڲ���ȫ���������ӣ�����������У�����XCl��������Һ���ղ������ⶨCO�ĺ������仯ѧ��Ӧ���£�2XCl+2CO+2H2O�TX2Cl2•2CO•2H2O����1��X��̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��
��2��C��H��O����ԭ�ӵĵ縺���ɴ�С��˳��ΪO��C��H��
��3��X2Cl2•2CO2H2O��һ�������������ͼ1��ʾ��
����COΪ��Ϊ�ȵ��ӵķ�����N2��
�ڸ����������ԭ�ӵ��ӻ���ʽΪsp3�ӻ���
����X2Cl2•2CO.2H2O�У�ÿ��Xԭ����������ԭ���γ�3����λ������ͼ���á����������Ӧ����λ��
 ��
����4�������ӵ������IJⶨ�ж��ַ�����X�������䷨��������һ�֣�ͨ����XCl�����X��������ͼ��ķ��������Եó�XCl�ľ�����ͼ2��ʾ�������ÿ��X+�����Cl-�ĸ���Ϊ4����Xԭ�ӵİ뾶Ϊa pm��������ܶ�Ϊpg/cm3����ͨ�����㰢���ӵ�����NA=$\frac{4����64+45.5��}{��4a��\frac{\sqrt{2}}{2}��1{0}^{-10}��^{3}p}$���м���ʽ���
���� ��1��Ԫ��X�Ļ�̬ԭ���еĵ��ӹ���7���ܼ���������������Ϊ1��Xԭ�ӵ��ڲ���ȫ���������ӣ���������Ų�ʽΪ1s22s22p63s23p63d104s1����XΪCu��
��2���ǽ�����Խǿ���縺��Խ��
��3����ԭ��������ȡ��۵���������ȵ�����Ϊ�ȵ����壻
�ڸ���ͼʾ�۲죬ÿ����ԭ����7��������ͭ��1�������гɹ��ۼ�������һ��ͭԭ���γ���λ��������4���۵��Ӷԣ�
��̼�������й¶Ե��ӣ���Cu֮���γ���λ����ÿ����ԭ����ͭԭ���γɹ��ۼ�������һ��ͭԭ���γ���λ����
��4��ͼʾ�����в��ܹ۲쵽��ɵ�X�ɼ���������Clԭ�ӵijɼ�����ж�X�����ͼ�й۲쵽ÿ��Clԭ����Χ��4��X�����������XCl������Ϊ1��1���ʾ���ÿ��X+�����Cl-�ĸ�����4����
��������λ�õ�X+��Խ����ϵ�X+���ڣ���Xԭ�ӵİ뾶Ϊa pm�����ⳤΪ4a��$\frac{\sqrt{2}}{2}$��10-10 cm���������㾧�������������Clԭ����ĿΪ4��CuCl�����Ӹ�����Ϊ1��1����Cuԭ����ĿΪ4���������㾧���������ٸ���m=��V���㣮
��� �⣺��1��Ԫ��X�Ļ�̬ԭ���еĵ��ӹ���7���ܼ���������������Ϊ1��Xԭ�ӵ��ڲ���ȫ���������ӣ���������Ų�ʽΪ1s22s22p63s23p63d104s1����XΪCu��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��
��2���ǽ�����Խǿ���縺��Խ������ΪO��C��H��
�ʴ�Ϊ��O��C��H��
��3����ԭ��������ȡ��۵���������ȵ�����Ϊ�ȵ����壬CO�ĵȵ��������ΪN2��
�ʴ�Ϊ��N2��
�ڸ���ͼʾ�۲죬ÿ����ԭ����7��������ͭ��1�������гɹ��ۼ�������һ��ͭԭ���γ���λ��������4���۵��Ӷԣ���Clԭ�Ӳ�ȡΪsp3�ӻ���
�ʴ�Ϊ��sp3�ӻ���
��̼�������й¶Ե��ӣ���Cu֮���γ���λ����ÿ����ԭ����ͭԭ���γɹ��ۼ�������һ��ͭԭ���γ���λ�����á����������Ӧ����λ��Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
��4��ͼʾ�����в��ܹ۲쵽��ɵ�X�ɼ���������Clԭ�ӵijɼ�����ж�X�����ͼ�й۲쵽ÿ��Clԭ����Χ��4��X�����������XCl�����Ӹ�����Ϊ1��1���ʾ���ÿ��X+�����Cl-�ĸ�����4����
��������λ�õ�X+��Խ����ϵ�X+���ڣ���Xԭ�ӵİ뾶Ϊa pm�����ⳤΪ4a��$\frac{\sqrt{2}}{2}$��10-10 cm���ʾ������Ϊ��4a��$\frac{\sqrt{2}}{2}$��10-10 cm��3��������Clԭ����ĿΪ4��CuCl�����Ӹ�����Ϊ1��1����Cuԭ����ĿΪ4��������Ϊ4��$\frac{��64+45.5��g/mol}{{N}_{A}}$����4a��$\frac{\sqrt{2}}{2}$��10-10 cm��3��pg/cm3=4��$\frac{��64+45.5��g/mol}{{N}_{A}}$�������ã�NA=$\frac{4����64+45.5��}{��4a��\frac{\sqrt{2}}{2}��1{0}^{-10}��^{3}p}$mol-1��
�ʴ�Ϊ��4��$\frac{4����64+45.5��}{��4a��\frac{\sqrt{2}}{2}��1{0}^{-10}��^{3}p}$��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����縺�ԡ��ȵ����塢�ӻ��������λ������������ȣ���4���о�������Ϊ�״��㡢�ѵ㣬��Ҫѧ������һ���Ŀռ���������ѧ����������

| A�� | 8�����ӵ�̼ԭ�ӵĺ��ط��ţ�12C | B�� | ��ϩ���ӵĽṹ��ʽ��CH2=CH2 | ||
| C�� | Cl-���ӵĽṹʾ��ͼ��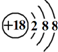 | D�� | CH4���ӵ����ģ�ͣ�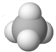 |
| A�� | C50��N70��C120��C540�Ȼ���Ϊͬ�������� | |
| B�� | CH3CH2CH2CH2OH�������ǣ����� | |
| C�� | 2-��ϩ�Ľṹ��ʽ��CH3CH2CH=CHCH3 | |
| D�� | ������Ϊ94��������Ϊ144���У�Pu��ԭ�ӣ�${\;}_{92}^{144}$Pu�� |
| A�� | NA���������ӵ�������1molC4H10�к����ۼ���ĿΪ14NA | |
| B�� | ij��Ӧ�ġ�H=-88kJ•mol-1��������Ӧ���һ��С��88kJ•mol-1 | |
| C�� | ��֪ij�¶��£�Kw=l��10-13������pH=8��NaOH��Һ��pH=5��H2SO4��Һ��ϱ����¶Ȳ��䣬��ʹ�����ҺpH=7����NaOH��Һ��H2SO4��Һ�������Ϊ11��9 | |
| D�� | ��Ũ��Ϊ0.1 mol•L-1 HF��Һ��ˮ����ϡ�����У�����Ⱥ�Ka��HF�����ֲ��䣬$\frac{c��{F}^{-}��}{c��{H}^{+}��}$ʼ�ձ������� |
| A�� | ��NaAlO2��Һ��ͨ�����CO2��2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- | |
| B�� | ��������Һ������������̼��Ӧ��2C6H5O-+CO2+H2O��2 C6H5OH+CO32- | |
| C�� | ��̼��������Һ�м������������������Һ��Ba2++OH-+HCO3-�TBaCO3��+H2O | |
| D�� | ���Ȼ�����Һ�ͷ�ˮ��Ӧ��ȡ�����������壺Fe3++3H2O����ˮ���TFe��OH��3��+3H+ |
| A�� | ŨH2SO4��ǿ�����ԣ�����������Cu�������ҷ�Ӧ | |
| B�� | ͭ�������γ����ܵ�����Ĥ | |
| C�� | ��CO2ͨ����������Һ�����ɴ����� | |
| D�� | �ⶨNaOH�۵�ʱ�����Խ�NaOH����ʯӢ�����и��¼��� |

 �״�������ȡ��ȩ��ԭ��ΪCH3OH��g��?HCHO��g��+H2��g����ij����С����1L�����ܱ������г���1molCH3OH���Ը÷�Ӧ������һϵ�е��о����õ��״���ƽ��ת�������¶ȵı仯������ͼ��ʾ��
�״�������ȡ��ȩ��ԭ��ΪCH3OH��g��?HCHO��g��+H2��g����ij����С����1L�����ܱ������г���1molCH3OH���Ը÷�Ӧ������һϵ�е��о����õ��״���ƽ��ת�������¶ȵı仯������ͼ��ʾ�� ����Ҫ�����1molij������A���ȼ�պ���Եõ�8molCO2��4molH2O������A�ڲ�ͬ�������ܷ���������ʾ��һϵ�б仯��
����Ҫ�����1molij������A���ȼ�պ���Եõ�8molCO2��4molH2O������A�ڲ�ͬ�������ܷ���������ʾ��һϵ�б仯��
 �ڵķ�Ӧ���ͣ���ȥ��Ӧ
�ڵķ�Ӧ���ͣ���ȥ��Ӧ +CH3COOH$��_{��}^{ŨH_{2}SO_{4}}$
+CH3COOH$��_{��}^{ŨH_{2}SO_{4}}$ +H2O����Ӧ���ͣ�������ȡ������Ӧ
+H2O����Ӧ���ͣ�������ȡ������Ӧ