��Ŀ����
7��ijNiO�ķ�������FeO��CuO��Al2O3��MgO��SiO2�����ʣ��ô˷�����ȡNiSO4��Ni���������£�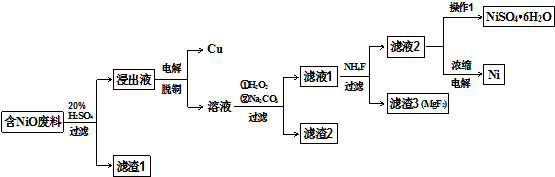
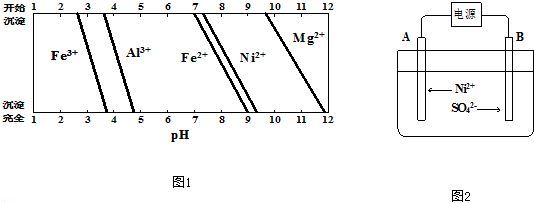
��֪���йؽ���������������������������pH��ͼ1��
��1������1����Ҫ�ɷ�ΪSiO2��
��2�������ͭ������ͭ������������
��3���������ӷ���ʽ���ͼ���H2O2������2H++H2O2+2Fe2+�T2Fe3++2H2O��
�ڼ�Na2CO3������Һ��pH��5��������2����Ҫ�ɷ�ΪFe��OH��3��Al��OH��3��
��4������Һ2�л��NiSO4��6H2O��ʵ������Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��5�����Ũ�������Һ2�ɻ�ý��������������Ӧԭ��ʾ��ͼ2���£�
��A���ĵ缫��ӦʽΪNi2++2e-�TNi��2H++2e-=H2����
��B������pH���С�����������С�����䡱������ƽ���ƶ�ԭ������B������pH�仯��ԭ��H2O?H++OH-��OH-��B���ŵ�ʹc��OH-�����ͣ�ƽ�������ƶ���c��H+��������pH���ͣ�
����һ��ʱ�����A��B�������ռ���11.2L���壨��״���£����������ܵõ�Ni29.35 g��
���� ijNiO�ķ�������FeO��CuO��Al2O3��MgO��SiO2�����ʣ�����ϡ�����ܽ����˵õ�����1ΪSiO2����ҺΪNiSO4��FeSO4��CuSO4��Al2��SO4��3����MgSO4������Һͨ�����ͭ���ӵõ���������ͭ����Һ����ͭ�����Һ�м���������������������ӣ��ټ���̼������Һ������ҺPH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ1�м���NH4F����Mg2+�����ɳ�������3ΪMgF2�����˵õ�����Һ2����Һ2�л��NiSO4��6H2O����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬���Ũ�������Һ2�ɻ�ý�������
��1������������֪������1Ϊ�������裻
��2�������ͭ��ͭ�����������ϵõ����ӷ�����ԭ��Ӧ������ͭ��
��3���ټ���H2O2����������������Һ��������������Ϊ�����ӣ�
�ڷ�������������ȫ��������ҺPH����Na2CO3������Һ��pH��5��ʹ�����ӣ�������ȫ�����������˵õ�����2��
��4����Һ�еõ����ʾ���ķ��������������ܽ�����¶ȱ仯��ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ��ᾧˮ���
��5�������ݵ���ԭ���������������Һ������������ĵ缫AΪ���ص���������Һ�������ӡ������ӵõ����ӷ�����ԭ��Ӧ�����������������������������ĵ缫BΪ��������Һ������������ʧ���ӷ���������Ӧ��
�����������������ĵ缫BΪ��������Һ������������ʧ���ӷ���������Ӧ���缫������ˮ����ƽ�ⱻ�ƻ���������Ũ������
�����ݵ缫��Ӧ�͵����غ����õ�����������
��� �⣺������ȡNiSO4��Ni������Ϊ��ijNiO�ķ�������FeO��CuO��Al2O3��MgO��SiO2�����ʣ�����ϡ�����ܽ����˵õ�����1ΪSiO2����ҺΪNiSO4��FeSO4��CuSO4��Al2��SO4��3����MgSO4������Һͨ�����ͭ���ӵõ���������ͭ����Һ����ͭ�����Һ�м���������������������ӣ��ټ���̼������Һ������ҺPH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ1�м���NH4F����Mg2+�����ɳ�������3ΪMgF2�����˵õ�����Һ2����Һ2�л��NiSO4��6H2O����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬���Ũ�������Һ2�ɻ�ý�������
��1������������֪������1��Ҫ�ɷ�Ϊ�������裬�ʴ�Ϊ���������裻
��2�������ͭ��ͭ���������������������ϵõ����ӷ�����ԭ��Ӧ������ͭ��Cu2++2e-=Cu���ʴ�Ϊ������
��3���ټ���H2O2����������������Һ��������������Ϊ�����ӣ�������ҺPH���ڳ������������ⷢ����Ӧ�����ӷ���ʽΪ��2H++H2O2+2Fe2+�T2Fe3++2H2O��
�ʴ�Ϊ��2H++H2O2+2Fe2+�T2Fe3++2H2O��
����ͼ1������������ȫ��������ҺPH����Na2CO3������Һ��pH��5����ʱ��Һ�������ӣ�������ȫ�����������Թ��˵õ�����2����Ҫ�ɷ�ΪFe��OH��3��Al��OH��3��
�ʴ�Ϊ��Fe��OH��3��Al��OH��3��
��4����Һ�еõ����ʾ���ķ��������������ܽ�����¶ȱ仯��ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ��ᾧˮ�������Һ2�л��NiSO4��6H2O��ʵ������Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��5������ͼ2��֪���������Һ������������ĵ缫AΪ���ص���������Һ�������ӡ������ӵõ����ӷ�����ԭ��Ӧ���������������缫��ӦΪ��Ni2++2e-�TNi��2H++2e-=H2����
�ʴ�Ϊ��Ni2++2e-�TNi��
�����������������ĵ缫BΪ��������Һ������������ʧ���ӷ���������Ӧ���缫������ˮ����ƽ�ⱻ�ƻ���H2O?H++OH-��OH-��B���ŵ�ʹc��OH-�����ͣ�ƽ�������ƶ���c��H+����������ҺPH��С��
�ʴ�Ϊ����С��H2O?H++OH-��OH-��B���ŵ�ʹc��OH-�����ͣ�ƽ�������ƶ���c��H+��������pH���ͣ�
�����ݵ缫��Ӧ�͵����غ����õ�����������
��3��һ��ʱ�����A��B�������ռ���11.2L���壨��״���£����������ʵ���=$\frac{11.2L}{22.4L/mol}$=0.5mol��
�����缫��Ӧ��Ni2++2e-�TNi
1mol 0.5mol
2H++2e-=H2����
1mol 0.5mol
�����缫��ӦΪ4OH--4e-=2H2O+O2��
2mol 0.5mol
����Ni���ʵ���Ϊ0.5mol������=0.5mol��58.7/mol=29.35g��
�ʴ�Ϊ��29.35��
���� ���⿼�������ʷ�����Ʊ����̵ķ����жϣ���Ҫ��ʵ����������͵���ԭ���ķ���Ӧ�ã��������ʺ͵缫��Ӧ��д�������غ�ļ���Ӧ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��Ӧԭ����
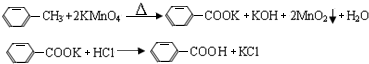
��Ӧ�Լ������������������
| ���� | ��Է������� | ��״ | �۵� | �е� | �ܶ� | �ܽ�� | ||
| ˮ | �Ҵ� | ���� | ||||||
| �ױ� | 92 | ��ɫҺ����ȼ�ӷ� | -95 | 110.6 | 0.8669 | ���� | �� | �� |
| ������ | 122 | ��ɫƬ״����״���� | 122.4 | 248 | 1.2659 | �� | ���� | ���� |

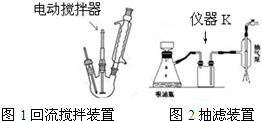
ʵ�鷽����һ�����ļױ���KMnO4��Һ����ͼ1װ���У���100��ʱ����Ӧһ��ʱ�䣬��ֹͣ��Ӧ�����������̷����������ͻ���δ��Ӧ�ļױ���
��1��ͼ2����K������Ϊ��ȫƿ����ɫҺ��A�Ľṹ��ʽΪ
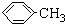 ������������
��������Ϊ������2�������Һ����ɫ��Ҫ�ȼ���������أ�Ȼ���ټ���Ũ�����ữ������������ص�Ŀ���dz�ȥδ��Ӧ�ĸ�������������������������ữʱ�ᷢ�����ᱻ�������������������������
��3�����й�����������װ����ʹ����ȷ����ABD��
A�����˿��Լӿ�����ٶȣ��õ��ϸ���ij���
B����װ�綯������ʱ��������¶˲�����������ƿ�ס��¶ȼƵȽӴ�
C�����˽�����Ϊ��ֹ������Ӧ�ȹر�ˮ��ͷ���ٶϿ����ϵͳ�����ϵͳ������
D����������ˮ���������½��ϳ�
��4����ȥ�����ڱ������еļױ�Ӧ�ȼ���NaOH��Һ����Һ��ˮ���ټ���Ũ�����ữ��Ȼ����ˣ����T�ɵõ������ᣮ
��5�����Ȳⶨ����ȡ1.220g��Ʒ�����100mL��Һ��ȡ����25.00mL��Һ�����еζ�������KOH���ʵ���Ϊ2.4��10-3mol����Ʒ�б�������������Ϊ96%��
| A�� | �������̵ķ�Ӧ�����ɷ�Ӧ�ھ��� | B�� | N2O2��N2O�Ǹ÷�Ӧ�Ĵ��� | ||
| C�� | ��$\frac{1}{2}$v��NO��=v��N2��ʱ����Ӧ�ﵽƽ�� | D�� | �÷�Ӧ�Ļ��Ϊ665kJ•mol-3 |
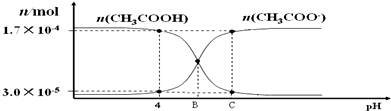
| A�� | ����ĵ��볣���ı���ʽ����Ka=$\frac{c��C{H}_{3}CO{O}^{-}��•c��{H}^{+}��}{c��C{H}_{3}COOH��}$ | |
| B�� | pH=4 ʱ������ĵ��볣�� Ka��1.8��10-5 | |
| C�� | B��ʱ����Һ�е����̪��Һ���Ժ�ɫ | |
| D�� | C��ʱ��c��CH3COO������c��Na+����c��H+����c��OH���� |
 A��B��C��D��E��F����Ԫ��Ϊԭ��������������Ķ�����Ԫ�أ�AΪԭ�Ӱ뾶��С��Ԫ�أ�A��B���γ�4ԭ��10���ӵķ���X��C���������������ڲ��3���� Dԭ�ӵ����������������ڲ��������һ�룻E�ǵؿ��к������Ľ���Ԫ�أ�FԪ�ص��������������۴�����Ϊ6����ش��������⣺
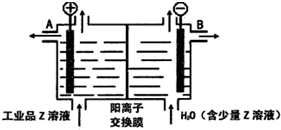
A��B��C��D��E��F����Ԫ��Ϊԭ��������������Ķ�����Ԫ�أ�AΪԭ�Ӱ뾶��С��Ԫ�أ�A��B���γ�4ԭ��10���ӵķ���X��C���������������ڲ��3���� Dԭ�ӵ����������������ڲ��������һ�룻E�ǵؿ��к������Ľ���Ԫ�أ�FԪ�ص��������������۴�����Ϊ6����ش��������⣺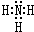 ��D������Һ̬X�з�����������A2C�ķ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ2Na+2NH3=2NaNH2+H2����
��D������Һ̬X�з�����������A2C�ķ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ2Na+2NH3=2NaNH2+H2����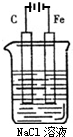 ��ͼ�ǵ��NaCl��Һ��ʾ��ͼ����ش��������⣺
��ͼ�ǵ��NaCl��Һ��ʾ��ͼ����ش��������⣺
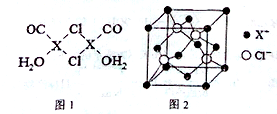 Ԫ��X�Ļ�̬ԭ���еĵ��ӹ���7���ܼ���������������Ϊ1��Xԭ�ӵ��ڲ���ȫ���������ӣ�����������У�����XCl��������Һ���ղ������ⶨCO�ĺ������仯ѧ��Ӧ���£�2XCl+2CO+2H2O�TX2Cl2•2CO•2H2O��
Ԫ��X�Ļ�̬ԭ���еĵ��ӹ���7���ܼ���������������Ϊ1��Xԭ�ӵ��ڲ���ȫ���������ӣ�����������У�����XCl��������Һ���ղ������ⶨCO�ĺ������仯ѧ��Ӧ���£�2XCl+2CO+2H2O�TX2Cl2•2CO•2H2O��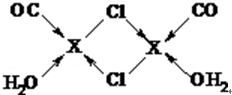 ��
��