��Ŀ����
14��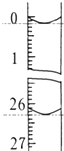 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ��������д���пհף���1���жϵζ��յ��������Һǡ����dz��ɫ�����ɫ���Ұ�����ڲ��ָ���
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���D
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ��������������Һ�����Ϊ26.10ml��
��4��ijѧ������3��ʵ��ֱ��¼�й����������
| �ζ� ���� | ����NaOH��Һ�����/mL | 0.1000mol/L��������/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 26.15 |
| �ڶ��� | 25.00 | 0.56 | 30.30 |
| ������ | 25.00 | 0.20 | 26.35 |
�������ϱ����ݼ����NaOH��Һ�����ʵ���Ũ��0.1046mol/l ��������ȡ4λ��Ч������
���� ��1���ζ��յ�������ƿ����Һ����ɫ�仯�жϣ�
��2������c���=$\frac{c���ᣩ��V���ᣩ}{V���}$��������������
��3����ͼ��֪����ʼV=0.20mL������ʱV=26.30mL��
��4���ٸ�������������Һ���������ƽ��ֵ��
���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ������c���=$\frac{c���ᣩ��V���ᣩ}{V���}$������Ũ�ȣ�
��� �⣺��1���������̪��죬�ζ��յ�ʱ�۲���ƿ����Һ����ɫdz��ɫ�����ɫ���Ұ�����ڲ��ָ�����ﵽ�ζ��յ㣬
�ʴ�Ϊ����Һǡ����dz��ɫ�����ɫ���Ұ�����ڲ��ָ���
��2����c���=$\frac{c���ᣩ��V���ᣩ}{V���}$��֪��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������ᣬ����������������c���ƫ��A��ѡ��
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и����Ӱ�죬��B��ѡ��
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ������������������c���ƫ��C��ѡ��
D����ȡ�������ʱ����ʼ���Ӷ���������ƫС���ζ�����ʱ���Ӷ���������ƫ�����ƫС��������������ƫС������c���ƫ�ͣ���Dѡ��
�ʴ�Ϊ��D��
��3����ͼ��֪����ʼV=0.20mL������ʱV=26.30mL��������������Һ�����Ϊ26.30mL-0.20mL=26.10mL���ʴ�Ϊ��26.10��
��4����������������Һ�������֪��ƽ��ֵΪ$\frac{26.15+��30.30-0.56��+��26.35-0.20��}{3}$=27.35mL���ʴ�Ϊ��27.35��
�ڸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=26.15mL����c���=$\frac{c���ᣩ��V���ᣩ}{V���}$��֪��c���=$\frac{0.1000mol/L��0.02615L}{0.025L}$=0.1046mol/l��
�ʴ�Ϊ��0.1046mol/l��
���� ���⿼���к͵ζ�����ȷ�ζ�ʵ���е����������ݴ������к͵ζ����������ǽ����Ĺؼ���ע������к͵�ʵ�ʣ���Ŀ�Ѷ��еȣ�

��CO2 ��NH3 ��N2 ��Cl2��O3��H2 ��NO2��
| A�� | �٢ڢۢܢ� | B�� | ȫ�� | C�� | �ڢܢݢ� | D�� | �٢ܢݢ� |
 �����������н�����ʹ���ã��ǽ���ʹ�õĽ�����ʹҩ���С����ȱ���֮�ƣ����Ʊ�ԭ�����£�
�����������н�����ʹ���ã��ǽ���ʹ�õĽ�����ʹҩ���С����ȱ���֮�ƣ����Ʊ�ԭ�����£�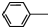 NH2+CH3COOH$\stackrel{��}{?}$
NH2+CH3COOH$\stackrel{��}{?}$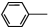 NHCOOCH3+HO
NHCOOCH3+HO��֪��
�ٱ����ױ�������
�����������������ʹ���IJ��������������±���
| ���� | �۵� | �е� | �ܽ�� |
| �������� | 114.3�� | 305�� | ������ˮ����������ˮ |
| ���� | -6�� | 184.4�� | ����ˮ |
| ���� | 16.6�� | 118�� | ������ˮ |
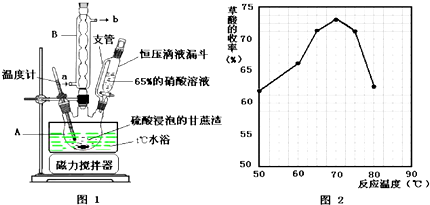
����1����a�У�����9mL ��0.10mol��������15mL��0.27mol�������ἰ����п�ۣ�������ͼװ����װ������
����2�������¶ȼƶ�����105�����ң�С����Ȼ�������Ӧ��ȫ��
����3�����Ƚ���Ӧ����ﵹ��ʢ��100mL ��ˮ���ձ��У���ȴ����ˣ�һ�ֿ��ٹ��˷�������ϴ�ӣ��õ��ֲ�Ʒ��
����4��������3���ôֲ�Ʒ��һ���ᴿ�Ƶò�Ʒ����Ϊ10.8g��
��ش��������⣺
��1������a������ΪԲ����ƿ����ѡ����a����ѹ����B������ţ���
A.25mL B.50mL C.100mL D.250mL
��2��ʵ���м�������п�۵�Ŀ���Ƿ�ֹ������������ͬʱ���ŷ�ʯ�����ã�
��3������2�У������¶ȼƶ�����105�����ҵ�ԭ�����¶ȹ��Ͳ���������Ӧ�����ɵ�ˮ���¶ȹ���δ��Ӧ������������
��4���жϷ�Ӧ�ѻ�����ȫ�ķ���Ϊ��ƿ������ˮ���ӣ�
��5������3�г��Ƚ�����ﵹ��ʢ����ˮ���ձ��У������ȡ���ԭ������ȴ���������ճ��ƿ���ϲ��״�����
��6������4�дֲ�Ʒ��һ���ᴿ�����ᴿ�������ؽᾧ��
��7������ʵ��IJ���Ϊ80%��
| A�� | ˮ���ɱ����ۻ� | B�� | ʳ�κͱ������ۻ� | ||
| C�� | Һ���Һ�������� | D�� | ������ռ���ۻ� |
| A�� | c��Z��=0.45mol/L | B�� | c��Y2��=0.5mol/L | C�� | c��X2��=0 | D�� | c��X2��=0.5mol/L |
 ��
��