��Ŀ����
5��ͭ��Cu����һ����Ҫ�ij����������ִ����������ĵ�·�벻������Cu�Ļ������ڿ�ѧ�о���ҵ�����о���������;����CuSO4��Һ���������Һ�����Һ�ȣ���ش��������⣺��1����CuSO4��Һ����μ��백ˮ������������ɫ�����Ȼ��õ�����ɫ��Һ������ɫ������õ�����ɫ��Һ�����ӷ���ʽΪCu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��д��������ɫ�����ܷ�ӳ������ۼ��Ľṹ��ʽ
 ��
����2��Ԫ�ؽ�Au���������ڱ��еĵ������ڣ���Cuͬ�壮һ��ͭ��Ͻ�������������ܶѻ��Ľṹ���ھ�����Cuԭ�Ӵ������ġ�Auԭ�Ӵ��ڶ���λ�ã���úϽ���Cuԭ����Auԭ������֮��Ϊ3��1���þ����У�ԭ��֮����������ǽ�������
��3������������д���ܣ���ԭ�ӿɽ��뵽��Cuԭ����Auԭ�ӹ��ɵ��������϶�У�����Cuԭ����Auԭ�ӵ�ͬ�������þ��崢�������ѧʽӦΪCu3AuH8��
���� ��1������ͭ�ȺͰ�ˮ��Ӧ����������ͭ��������ͭ�Ͱ�ˮ��Ӧ�����������ݷ�Ӧд�����ӷ�Ӧ����ʽ��[Cu��NH3��4]2+���γ�4����λ�������жԳƵĿռ乹�ͣ�
��2�����þ�̯���������ֽ���ԭ�Ӹ���֮�ȣ�����������ԭ�Ӽ�ͨ����������ϣ�
��3��Hԭ��Ӧλ�ھ����ڲ���ÿ����ԭ������3��ͭԭ��һ����ԭ�ӣ�����Auԭ��λ�ڶ��㣬��8��Auԭ������8����ԭ�ӣ�
��� �⣺��1����ˮ������ͭ��Ӧ����������ͭ��ɫ����������ˮ����ʱ����ˮ��������ͭ��Ӧ���ɿ����Ե�ͭ������������ɫ������õ�����ɫ��Һ�����ӷ���ʽΪCu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��[Cu��NH3��4]2+���γ�4����λ�������жԳƵĿռ乹�ͣ���ṹ��ʽΪ�� ��
��
�ʴ�Ϊ��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-�� ��
��
��2���ھ����У�Auԭ��λ�ڶ��㣬Cuԭ��λ�����ģ��þ�����Auԭ�Ӹ���=8��$\frac{1}{8}$=1��Cuԭ�Ӹ���=6��$\frac{1}{6}$=3�����ԸúϽ���Cuԭ����Auԭ�Ӹ���֮��=3��1��ͭ��Ͻ����ڽ������壬����������ԭ�Ӽ�ͨ����������ϣ�
�ʴ�Ϊ��3��1����������
��3����ԭ�ӿɽ��뵽��Cuԭ����Auԭ�ӹ��ɵ��������϶�У���Hԭ��Ӧλ�ھ����ڲ���ÿ����ԭ������3��ͭԭ��һ����ԭ�ӣ�����Auԭ��λ�ڶ��㣬��8��Auԭ������8����ԭ�ӣ����Ըþ����к���8����ԭ�ӣ����仯ѧʽΪ��H8AuCu3��
�ʴ�Ϊ��Cu3AuH8��
���� ���⿼����λ�������������ļ���ȣ���Ŀ�Ѷ��еȣ�ע�������λ�����γ�ԭ���Լ���̯���ھ��������е�Ӧ�ã������ڿ���ѧ���ķ��������ͼ���������

| A�� | �÷�Ӧ�Ļ�ѧ����ʽΪCO+H2O$\frac{\underline{\;����\;}}{����}$CO2+H2 | |
| B�� | �÷�Ӧ���ʱ�Ϊ��ֵ | |
| C�� | ���º����£����뵪����ƽ������ | |
| D�� | �����¶ȣ��淴Ӧ���ʼ�С |
| A�� | �ɷ����ķ�Ӧ�����У���ȡ�� �ڼӳ� ����ȥ ������ ��ˮ�� ������ ���к� | |
| B�� | ������1mol������NaOH��Һ��Ӧ������NaOH�����ʵ���Ϊ2mol | |
| C�� | �����Ȼ�����Һ������ɫ��Ӧ | |
| D�� | ���л������ڷ����� |
| A�� | ����������Ӧ | B�� | ������ķֽ� | ||
| C�� | �Ҵ��������������Ӧ | D�� | �����������ķ�Ӧ |
| A�� | �������ơ��������ơ������İ���ͭ[Cu��NH3��4]SO4���⻯�� | |
| B�� | ����李��⻯李���������������[Ag��NH3��2]OH������� | |
| C�� | ���ᡢ�������ơ������ơ��������� | |
| D�� | �Ȼ�李��������ơ�˫��ˮ���������� |
| A�� |  | |
| B�� | CH3CH2CH2CHOHCH3$��_{��}^{Ũ����}$CH3CH2CH=CHCH3+H2O | |
| C�� | 2CH3CH2OH$��_{140��}^{ŨH_{2}SO_{4}}$CH3CH2OCH2CH3+H2O | |
| D�� | CH3COOH+CH3OH $��_{��}^{ŨH_{2}SO_{4}}$ CH3COOCH3+H2O |
 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ��������д���пհף���1���жϵζ��յ��������Һǡ����dz��ɫ�����ɫ���Ұ�����ڲ��ָ���
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���D
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ��������������Һ�����Ϊ26.10ml��
��4��ijѧ������3��ʵ��ֱ��¼�й����������
| �ζ� ���� | ����NaOH��Һ�����/mL | 0.1000mol/L��������/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 26.15 |
| �ڶ��� | 25.00 | 0.56 | 30.30 |
| ������ | 25.00 | 0.20 | 26.35 |
�������ϱ����ݼ����NaOH��Һ�����ʵ���Ũ��0.1046mol/l ��������ȡ4λ��Ч������

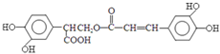
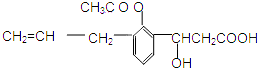
 $��_{-H_{2}O}^{��}$
$��_{-H_{2}O}^{��}$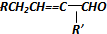
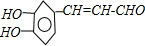 ��
��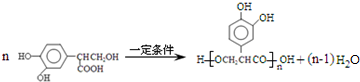 ��
�� ��
�� ��
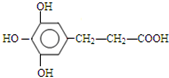
��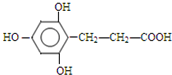 ��
��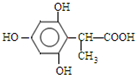 ����һ�֣���
����һ�֣���