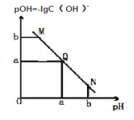
��Ŀ����
����Ŀ�������£���0.1 mol��L��1 NaOH��Һ�ζ�10 mL 0.1 mol��L��1 H2X��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ������˵������ȷ����

A.ˮ���������c(OH��)��D�㣾B��
B.C����ڹ�ϵʽ��c(Na��)��c(HX��)��c(X2��)��c(H��)
C.B�㣺c(HX��)��c(H��)��c(X2��)��c(H2X)
D.A����Һ�м�������ˮ��![]() ����
����
���𰸡�B
��������
A��B�㷴Ӧ������ΪNaHA��HA-�ĵ���̶ȴ�����ˮ��̶ȣ���Һ�����ԣ�������������ˮ�ĵ��룬��D�����20mL����������Һ������ǡ�÷�Ӧ����Na2A��A2-ˮ��ٽ���ˮ�ĵ��룬����ˮ�����c(OH-)��B�㣼D�㣬��A��ȷ��
B����Һ���ڵ���غ�c(Na+)+c(H+)=c(HX-)+2c(X-)+c(OH-)����c(Na+)=c(HX-)+c(X2-)+c(OH-)-c(H+)����B����
C��B��ʱ������10mLNaOH��Һ����Ӧ������ΪNaHA����ʱ��Һ��pHС��7��˵��HA-�ĵ���̶ȴ�����ˮ��̶ȣ���c(A2-)��c(H2A)�����������ӻ�����ˮ�ĵ��룬��c(H+)��c(A2-)����Һ������Ũ�ȴ�СΪ��c(HA-)��c(H+)��c(A2-)��c(H2A)����C��ȷ��
D������ͼ���֪��0.1mol/L��H2A��Һ��pH����1��˵��H2AΪ���ᣬ��A����Һ�м�������ˮ����Һ�������ӡ�H2A��Ũ�ȼ�С������ˮ�����ӻ����䣬������������Ũ����������![]() �ı�ֵ����D��ȷ��
�ı�ֵ����D��ȷ��
�ʴ�ΪB��

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�����Ŀ��X��Y��Z��Ϊ������Ԫ�أ���������Ϊ����Ԫ�أ�һ��Ϊ�ǽ���Ԫ�أ���ԭ�Ӱ뾶�ֱ�Ϊ
X | Y | Z | |
ԭ�Ӱ뾶/nm | 0.154 | 0.130 | 0.071 |
X��Y����ͬһ���ڣ�����Ԫ���γɵļ����Ӿ�����ͬ�ĵ��Ӳ�ṹ������˵����ȷ����
A. ԭ��������������Z>X>Y
B. ����Ԫ�ؿ���Ϊͬ����Ԫ��
C. ԭ��������Y>X>Z
D. ���Ӱ뾶��X>Y>Z
����Ŀ��̽����Һ����Զ�![]() ��Һ�����ƺͱ����Ӱ�졣10mL
��Һ�����ƺͱ����Ӱ�졣10mL![]() ��10mL
��10mL![]() ��Һ�С�
��Һ�С�
I.![]() ��Һ������
��Һ������
��![]() �ֱ�����10mL����ˮ��10mL
�ֱ�����10mL����ˮ��10mL
��Һ��� | �ܼ� | ��Һ��״ | |
�� | ����ˮ | dz��ɫ������Һ�� | �� |
�� |
| ��ɫ������Һ�� | �� |
�� |
| ��ɫ������Һ | �� |
(1)��ƽ���ƶ�ԭ�����͢������Ե���Ҫԭ����________________
(2)�ڡ��۱�췢����Ӧ�����ӷ���ʽ��_______________________
(3)��ͬѧ��Ϊ����һ����+3���������ü������������Һ��,�۲쵽��·:������KSCN�Ģ��е����Լ�a,��Һ���,˵�����ƶ���ȷ���Լ�a��____________
II ![]() ��Һ�ı���
��Һ�ı���
��ʵ��I�����Ƶ�������Һ�ֱ��ڿ����з���24Сʱ��,��¼���¡�
��Һ��� | ��Һ��״ | |
�� | ��ɫ���� | �� |
�� | ��ɫ��Һ | �μ�5�� |
�� | ��ɫ��Һ | �μ�5�� |
���������ۣ��٢�˵�����Լ���ʱ,![]() ��Һ���ױ��ʣ��ڢ�˵������
��Һ���ױ��ʣ��ڢ�˵������
���������ϣ���һ��pH��Χ�ڣ�+2�����Ļ�ԭ�������Լ�������ǿ,�����������������Ե���ǿ����ǿ��
������ʵ�飩����ͼ��ʾװ�����ʵ��(�μ��Լ�ʱ��Һ�������Һ�����Ա仯�ɺ���)�����ҳس����ȶ�ͨ������,��������ʾ���ȶ���:
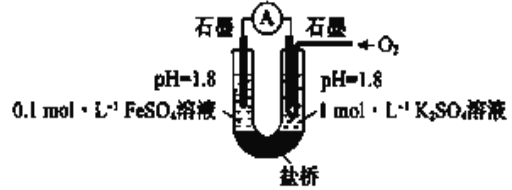
I ����صμ�Ũ������![]() �ӽ�
�ӽ�![]() ������û�����Ա仯
������û�����Ա仯
II ���ҳصμӵ���Ũ���ᣬ������������
(4)��ȫ���������ۣ�:�ڢ�˵��_______________
(5)ii���ҳصĵ缫��Ӧ����ʽ��____________________
(6)����ʵ���ƶϣ��۱Ȣ���![]() ���ױ��ʵ�ԭ����___________________
���ױ��ʵ�ԭ����___________________
(7)������ԭ����װ�����½���ʵ��֤ʵ����![]() ���ױ��ʵ�ԭ��ʵ�鷽����Ԥ��������:���ҳس����ȶ�ͨ������,��������ʾ���ȶ���____________
���ױ��ʵ�ԭ��ʵ�鷽����Ԥ��������:���ҳس����ȶ�ͨ������,��������ʾ���ȶ���____________
(8)����ʵ��,���Ʋ�����![]() ��Һ����ѷ���_________________��
��Һ����ѷ���_________________��