��Ŀ����
̼��þ������һ�����͵��������β����е���ǿ���ϡ�
��1���ϳɸ����ʵIJ������£�
����1������0.5mol��L-1MgSO4��Һ��0.5mol��L-1NH4HCO3��Һ��
����2������Ͳ��ȡ500mL NH4HCO3��Һ��1000mL�Ŀ���ƿ�У��������������¶ȿ�����50�档
����3����250mL MgSO4��Һ��μ���NH4HCO3��Һ�У�1min�ڵμ�����ð�ˮ������ҺpH��9.5��
����4������1h���ˣ�ϴ�ӡ�
����5����40�����ո������и���10h����̼��þ�����Ʒ��MgCO3��nH2O n=1~5����
�ٲ���2�����¶���50�棬�Ϻõļ��ȷ����� ��
�ڲ���3����MgCO3��nH2O���������ӷ���ʽΪ ��
�۲���4�����Ƿ�ϴ�Ӹɾ��ķ����� ��
��2���ⶨ�ϳɵ�MgCO3��nH2O�е�nֵ��
����1.000g̼��þ���룬������ͼ��ʾ�Ĺ��ƿ�м���ˮ����ϡ�����뾧�뷴Ӧ�����ɵ�CO2��NaOH��Һ���գ��������·�Ӧ4~5h����Ӧ���ڽ��¶�����30�棬�����ձ��е���Һ����֪Ũ�ȵ�����ζ������CO2���������ظ���������2�Ρ�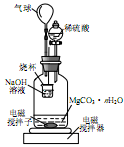
��ͼ������������� ��
��������Ӧ����Ҫ���µ�30�棬��ҪĿ���� ��
����3��ʵ����ÿ1.000g̼��þ���������CO2ƽ��ֵΪa mol����nֵΪ ���ú�a�ı���ʽ��ʾ����
��3����ȡ100g���������Ʒ�������ط���������������ͼ��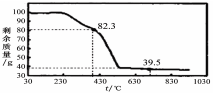
��������ºϳɵľ����У�n= ��ѡ�1��2��3��4��5����
��1����ˮԡ���� ��Mg2++HCO3-+NH3��H2O+(n-1)H2O=MgCO3��nH2O+NH4+
��ȡ���һ��ϴ��Һ���������������ữ��BaCl2��Һ���������ֳ�������ϴ�Ӹɾ�
��2����ƽ��ѹǿ
���¶����������ܽ�ȼ�С��ʹ���ƿˮ���ܽ�Ķ�����̼�����ݳ����������Ƴ�����գ�
�ۣ�1.000-84a��/18a
��3��1
���������������1����ˮԡ�������Ⱦ��ȣ����ڿ��ƣ�������100����ɲ���ˮԡ���ȣ���Mg2++HCO3-+NH3��H2O+(n-1)H2O=MgCO3��nH2O+NH4+ �۳��������в��������ͨ��ϴ��Һ�к��е�����������SO42-���ж��Ƿ�ϴ�Ӹɾ�����2���ٷ�Ӧ��ϵ�������壬��ֹѹǿ������Σ�գ���������ã����¶����������ܽ�ȼ�С��ʹ���ƿˮ���ܽ�Ķ�����̼�����ݳ����������Ƴ�����գ�
��n(MgCO3)=n(CO2)="a" mol
m(H2O)=(1.000-84a)g
n(H2O)=��1.000-84a��/18 mol
��n=��1.000-84a��/18a
��3����ͼ�п��Եó�MgCO3��nH2O���82.3gʱΪʧȥ�ᾧˮ�ķ�Ӧ�����õ�����MgO��
100��18n/��84+18n��=100-82.3 n=1
���㣺��������ɵIJⶨΪ���壬���黯ѧʵ����������ѡ��ϴ�Ӳ���������������ʵ�鷽������������۵��й����⡣

��֪��
| ҩƷ���� | �۵�/�� | �е�(��) | �ܶ�g/cm3 | �ܽ��� |
| ������ | -89.5 | 117.7 | 0.8098 | ����ˮ������Ũ���� |
| 1-�嶡�� | -112.4 | 101.6 | 1.2760 | ������ˮ��Ũ���� |
�����������������գ�
��һ���Ʊ�1���嶡��ֲ�Ʒ����ͼװ�õ�Բ����ƿ�����μ���NaBr��10 mL ��������2����ʯ����������1:1��������Һ��ҡ�ȣ�����30 min��

��1��д���Ʊ�1���嶡��Ļ�ѧ��Ӧ����ʽ��__________________________________________________
��2����Ӧװ���м����ʯ��Ŀ����__________________�����������Ϊ1:1���������õĶ�������Ϊ (ѡ���ţ���
a����ƽ b����Ͳ c������ƿ d���ζ���
��3����Ӧװ���У����˲����ˮ֮�⣬�����ܴ��� �� ���л������
��4������Ũ�������ʵ�飬�л����л�����ػ�ɫ����ȥ�������ʵ���ȷ������ (ѡ���ţ���
a������ b������������Һϴ��
c�������Ȼ�̼��ȡ d��������������Һϴ��
�������Ʊ���Ʒ
���õ��Ĵ�1-�嶡��������Ũ���ᡢˮ��10% ̼���ơ�ˮϴ�Ӻ������ˮ�Ȼ��ƽ��и��Ȼ���ٽ�1-�嶡�鰴ͼװ������

��5���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________�����ң�����1-�嶡�龫Ʒ�ʹ�Ʒ��һ�ַ�����____________________��
��6��ʵ���Ƶõ�1-�嶡�������Ϊ10.895 g������������ת����Ϊ ��������3λС������
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
| | �� | �� | �屽 |
| �ܶ�/g��cm��3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
�����кϳɲ���ش����⣺
(1)��a�м���15 mL��ˮ����������м����b��С�ļ���4.0 mLҺ̬�塣��a�е��뼸���壬�а�ɫ��������������Ϊ������_______���塣�����μ���Һ����ꡣװ��d��������_____ ___��
(2)Һ�����������в�������ᴿ��
����a�м���10 mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10 mLˮ��8 mL 10%��NaOH��Һ��10 mLˮϴ�ӡ�NaOH��Һϴ�ӵ�������________��
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˡ������Ȼ��Ƶ�Ŀ����____________________��
(3)�����Ϸ���������屽�л����е���Ҫ����Ϊ________��Ҫ��һ���ᴿ�����в����б������________(������ȷѡ��ǰ����ĸ)��
A���ؽᾧ B������ C������ D����ȡ
(4)�ڸ�ʵ���У�a���ݻ����ʺϵ���________ (������ȷѡ��ǰ����ĸ)��
A. 25 mL B��50 mL C��250 mL D��500 mL
����������(����ʽΪC4H2O4Fe���ṹ��ʽΪ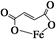 )��һ������ʹ�õ���ǿ������
)��һ������ʹ�õ���ǿ������
(1)��ͼΪʵ����ģ�ҵ��ȡ����������������ͼ��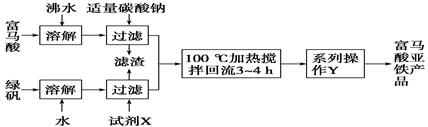
�ٸ������⣬����д��������Ľṹ��ʽ��________________________��
���̷�(FeSO4��7H2O)�ڱ���������γɵ�������Ҫ��________(�ѧʽ)��
�۲���Y����________����ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȡ�
���жϲ���Y�С�ϴ�ӡ�������������Ʒ������ϴ����ʵ�鷽����___________��
(2)���һ��ʵ�鷽����֤�����ø�����������Ʒ����������(�ɹ�ѡ�õ��Լ���KSCN��Һ��H2O2��Һ��ϡ���ᡣ)����д�±���Ӧ�ո�
| ���� | ʵ����������� |
| �� | ȡ������������Ʒ1.5 g������ϡ����25 mL����ˮϡ����50 mL������ʹ����ȫ�ܽⲢ��Ӧ����ȴ�����(��ȥ���ɵĸ����ἰ���ܹ����ķ�Ӧ��)��������Һ |
| �� | |
| �� | |
ij��ѧ��ȤС���ͬѧͨ�����²�����ȡ�����еĵ⣺
�ٽ�����ɹ�����ճɻ� �ڽ������ҽ��ݵ�������Һ �۹��˵ú������ӵ���Һ �������Һ�м�������Cl2�û����� �ݶԺ�����Һ����һϵ�е���ȡ��õⵥ��
��1�����չ��̿����������������н���_____________
| A���ձ� | B���Թ� | C�������� | D������ |
��3�����л��ܼ������ˮ����ȡ�������õ���������װ��

(4)�������Ȼ�̼���ܼ�������ȡ,��ֲ���²�����Ϊ
������Դ�����þ��й���ǰ����
��1�����辭����ѧ�仯���ܴӺ�ˮ�л�õ�������________������ţ�
| A��Cl2 | B����ˮ | C���ռ� | D��ʳ�� |
��3����ͼ�ǴӺ�ˮ����ȡþ�ļ����̡�

�ٹ�ҵ�ϳ����ڳ���Mg2�����Լ�A��________��
 ת��ΪMgCl2�����ӷ���ʽ��________________________________��
ת��ΪMgCl2�����ӷ���ʽ��________________________________��������ˮMgCl2��ȡMg�Ļ�ѧ����ʽ��________________________��
��4���������и�����I����ʽ���ڵĵ�Ԫ�ء�ʵ������ȡI2��;��������ʾ��

�����պ������ҽ�ʱ���õ���Ҫ����������________________��
�����ữ����Һ�мӹ���������Һ��д���÷�Ӧ�����ӷ���ʽ____________________________��
��Ӧ�������ټ���CCl4����ȡ���������ã����Թ۲쵽CCl4���________ɫ��
ij��ѧ��ȤС��ͬѧ���̡�֤�������д��ڵ�Ԫ�ء���ʵ��ʱ���Կα��ϵ����������������պ�Ļҽ��е�Ԫ����I-��ʽ���ڡ����������ʡ�����KI���屻�������ʣ���dz��ɫ����I2����KI����ֱ�Ӽ���ʱ��Ϊʲô���������أ���ˣ�С��ͬѧ���I-������������������֮���Ҫʲô����������ʵ��̽����
��������衿���ݾ���KI����ᱻ���������ʣ���Ͽ����ijɷ֣������ų� ��ϡ�������Ӱ�졣Ȼ���KI������������������裺
����һ����ҪH2O�IJ��룻
���������ҪCO2�IJ��룻
�������� ��
��ʵ����֤��
(1)Ϊ����֤�������С��ͬѧ���������ʵ�鷽����ȡ10mLKI��Һ(Ũ�Ƚϴ�)��5֧�Թ��У����Թ���ͨ��CO2������ߵμ����ᡣһ��ʱ���Ա��Թ��е���ɫ��dz��֮�����Թ��е��������Һ���ٴζԱ��Թ��е���ɫ��dz������ʵ��������±���
| �Թ���� | 1 | 2 | 3 | 4 | 5 |
| ͨ��CO2ʱ��/min | 0 | 1 | 5 | | |
| �����/�� | 0 | | | 3 | 6 |
| ��Һ����ɫ�Ա� | ��ɫ | dz��ɫ����ɫ�����μ�� | |||
| �μӵ�����Һ�����ɫ�Ա� | ��ɫ | dz��ɫ����ɫ�����μ�� | |||
С��ͬѧͨ��ʵ����������˼����CO2�ڷ�Ӧ�����е����ã��ó����ۣ�CO2����ͬ����һ����KI�������Ĺ������ṩ ������
д����ʵ����CO2��KI��Ӧ�Ļ�ѧ����ʽ ��
(2)Ϊ����֤I-�������Ƿ����Ҫ��H2O�IJ��룬С��ͬѧ���������ʵ����֤��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ��ȡһ�ݴ�����KI���壬�ֳ����ȷݣ� | |
| ��һ�ݼ��뵽װ�� �ļ���ƿA�У� һ�ݼ��뵽װ�� �ļ���ƿA�У� �ۼ����۲졣 | |
��������ߡ�
���ڿ�����CO2��ˮ�����ĺ����ܵͣ������ڼ���KI�Ĺ�������������������ױ�����ɢ�������ܺܺõ���KI�Ӵ���KI��Ȼ�Ͳ��ܱ���������ʱ�䱣��⻯����Ҫ�ܹ⡢ ��
���ͷ���һ�ֻ�ѧ���ɼ�������С�մ��ۣ�̼����泥�������[KAl(SO4)2?12H2O]�е�����������ɡ�ijС��Ϊ̽����ͬƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣���������ʵ�顣
��������衿
��1������1����С�մ�ͳ������
����2����С�մ���������
����3����__________________________���
�����������̡�
Ϊ̽����Ʒ�Ƶķ��ͷ۵ijɷ֣�ijͬѧ�������ʵ�飬�õ���������
��2����ϲ���١��۷���������AΪ________���÷��ͷ۵ijɷ�Ϊ__________________��
��3��������ٺ͢ڲ������䣨����Ҳ��ͬ�����������������ϡ�����Ϊ�����Ȼ�����Һ���۲쵽�а�ɫ�������ɣ��ܷ�ȷ�����ͷ۵ijɷֲ�˵�����ɣ�________________�� ____________________________________________________________________��
��4����Ʒ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2����������ʵ����֤��
ʵ����������Ʒ��ѡ����ѡ�Լ���ϡ���ᡢ0.1 mol/LNaOH��Һ��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ������Ʒ��������������� ����Һ�ֳ����ݣ��ֱ�װ��A��B�Թ��С� | |
| ����2��_____________________________ __________________________________ | ________________________֤����Na+���� �ͷ�����NaHCO3�� |
| ����3��_____________________________ ___________________________________ | ___________________________________ _______����ϲ���2�еĽ��ۣ�����2������ |
