��Ŀ����
����Ŀ������ʱ����֪Ka(CH3COOH)=Kb(NH3H2O)������Ũ�Ⱦ�Ϊ0.1mol��L-1�Ĵ���Ͱ�ˮ��������Һ��˵����ȷ���ǣ� ��
������Һ��pH֮��Ϊ14
������Һ�д����NH3H2O�ĵ���̶���ͬ
������Һ��ˮϡ�ͣ���Һ�и�����Ũ�Ⱦ�����
������Һ�������ϣ�pH=7����ˮ�����c(H+)=1��10-7mol��L-1
A.�٢�B.�ڢ�C.�٢ڢ�D.�٢ڢۢ�
���𰸡�A
��������
����ʱ��0.1mol��L-1�Ĵ���Ͱ�ˮ��Һ��Ka=![]() ��Kb=
��Kb=![]() ����֪Ka(CH3COOH)=Kb(NH3H2O)��c(CH3COOH)=c(NH3H2O)����c(H+)(CH3COOH)= c(OH-)(NH3H2O)��
����֪Ka(CH3COOH)=Kb(NH3H2O)��c(CH3COOH)=c(NH3H2O)����c(H+)(CH3COOH)= c(OH-)(NH3H2O)��
�������Ϸ���֪��c(H+)(CH3COOH)= c(OH-)( NH3H2O)����pH(CH3COOH)=pOH(NH3H2O)=14- pH(NH3H2O)������pH(CH3COOH)+ pH(NH3H2O)=14������ȷ��
������Һ�д����NH3��H2O����ʼŨ�ȶ�Ϊ0.1mol��L-1��c(H+)(CH3COOH)= c(OH-)( NH3H2O)�����Ե���̶���ͬ������ȷ��
������Һ��ˮϡ�ͣ�������Һ�е�c(OH-)����ˮ�е�c(H+)���۲���ȷ��
������Һ�������ϣ�pH=7����ʱ����CH3COONH4������˫ˮ�ⷴӦ���ٽ�ˮ�ĵ��룬������ˮ�����c(H+)>1��10-7mol��L-1���ܲ���ȷ��
�ۺ����Ϸ������٢���ȷ����ѡA��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�����Ŀ��ij��·��������ҵ��ˮ����������������ŷŵ���ˮ�����±���ʾ��Ϊ�о���ˮ��Cu2+���������pH��ȡ5�ݵ����ķ�ˮ���ֱ���30%��NaOH��Һ����pH��8.5��9��9.5��10��11�����ú����ϲ���Һ��ͭԪ�صĺ�����ʵ��������ͼ��ʾ���������ϣ�
ƽ���![]()
ƽ���![]()
��Ŀ | ��ˮˮ�� | �ŷű� |
pH | 1.0 | 6~9 |
Cu2+/mgL-1 | 72 | ��0.5 |
NH4+/mgL-1 | 2632 | ��15 |
����˵��������ǣ� ��
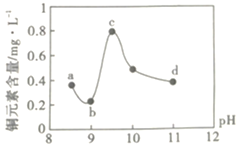
A.a~b�η����ķ�ӦΪ��![]()
B.b~c�Σ���pH���ߣ�Cu(OH)2�������ӣ�����ƽ��������ƶ���ͭԪ�غ�������
C.c~d�Σ���pH���ߣ�c(OH-)���ӣ�ƽ��������ƶ���ͭԪ�غ����½�
D.d���Ժ���c(OH-)���ӣ�ͭԪ�غ�����������
����Ŀ������ƽ�ⳣ��(��Ka��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
��ѧʽ | HF | H2CO3 | HClO |
����ƽ�� ����(Ka) | 7.2��10-4 | K1=4.4��10-7 K2=4.7��10-11 | 3.0��10-8 |
(1)��Ũ��Ϊ0.1mol��L-1HF��Һ��ˮϡ��һ��(�����¶Ȳ���)�����и����������___��
A��c(H+) B��c(H+)��c(OH-) C��![]() D��
D��![]()
(2)25��ʱ����20mL0.1mol��L-1������м���VmL0.1mol��L-1NaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����___��
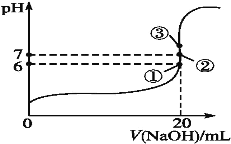
A��pH=3��HF��Һ��pH=11��NaF��Һ�У���ˮ�������c(H+)���
B���ٵ�ʱpH=6����ʱ��Һ��c(F-)-c(Na+)=9.9��10-7mol��L-1
C���ڵ�ʱ����Һ�е�c(F-)=c(Na+)
D���۵�ʱV=20mL����ʱ��Һ��c(F-)<c(Na+)=0.1mol��L-1
(3)���ʵ���Ũ�Ⱦ�Ϊ0.1mol��L-1������������Һ����Na2CO3��Һ����NaHCO3��Һ����NaF��Һ����NaClO��Һ�����������ж�pH�ɴ�С����___��
(4)Na2CO3��Һ�Լ�������Ϊ![]() ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤֮___��
ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤֮___��
(5)����������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO��ṹʽΪH-O-F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A��ÿ����1molHFת��___mol���ӡ�