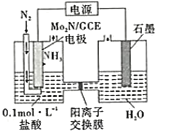
��Ŀ����
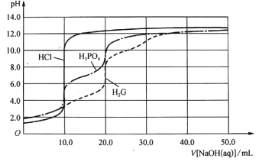
����Ŀ��25��ʱ����0.10molL-1�İ�ˮ�ζ�10.00mL0.05molL-1�Ķ�Ԫ��H2A����Һ���ζ������м��백ˮ�����(V)����Һ��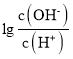 �Ĺ�ϵ��ͼ��ʾ������˵����ȷ����(����)
�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����(����)

A.H2A�ĵ��뷽��ʽΪH2AH++HA-
B.B����Һ�У�ˮ�������������Ũ��Ϊ1.0��10-6molL-1
C.C����Һ�У�c(NH4+)+c(NH3H2O)=2c(A2-)
D.25��ʱ����ˮ�ĵ���ƽ�ⳣ��Ϊ![]()
���𰸡�BD
��������
A��0.05molL-1�Ķ�Ԫ��H2A����Һ��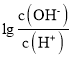 =-12��c(H+)c(OH-)=10-14����c(H+)=0.1mol/L=2c(H2A)��˵���ö�Ԫ����ȫ���룬����H2AΪǿ�ᣬ���뷽��ʽΪH2A=2H++A2-����A����
=-12��c(H+)c(OH-)=10-14����c(H+)=0.1mol/L=2c(H2A)��˵���ö�Ԫ����ȫ���룬����H2AΪǿ�ᣬ���뷽��ʽΪH2A=2H++A2-����A����
B��B����Һ���백ˮ10mL����Ӧǡ������(NH4)2A��笠�����ˮ�⣬��Һ�����ԣ���Һ��H+����ˮ�ĵ��룬��ʱ��Һ��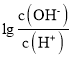 =-2����c(OH-)=10-2c(H+)��c(H+)c(OH-)=10-14������c(H+)=10-6mol/L����ˮ�������������Ũ��Ϊ1.0��10-6mol/L����B��ȷ��
=-2����c(OH-)=10-2c(H+)��c(H+)c(OH-)=10-14������c(H+)=10-6mol/L����ˮ�������������Ũ��Ϊ1.0��10-6mol/L����B��ȷ��
C��C����Һ�к���(NH4)2A��NH3H2O����Һ�����ԣ�c(H+)=c(OH-)�����ݵ���غ��c(NH4+)+c(H+)=c(OH-)+2c(A2-)������c(NH4+)=2c(A2-)����c(NH4+)+c(NH3H2O)��2c(A2-)����C����
D��C����Һ�����ԣ���c(H+)=c(OH-)=10-7 mol/L�����ݵ���غ��c(NH4+)=2c(A2-)=2��![]() mol/L=
mol/L=![]() mol/L�����������غ��c(NH3H2O)=
mol/L�����������غ��c(NH3H2O)=![]() mol/L-
mol/L-![]() mol/L=
mol/L=![]() mol/L����ˮ�ĵ��볣������ʽΪKb=
mol/L����ˮ�ĵ��볣������ʽΪKb=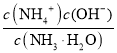 =
=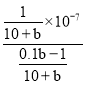 =
=![]() ����D��ȷ��
����D��ȷ��
��ѡBD��