��Ŀ����
14��ijУ��ѧѧ���о�С��������м��ϡ�����������Ʊ�Ħ���Σ���������泥�������ʵ�鲽�����£�A��Feм�Ĵ����ͳ���
��������ȡԼ5g��м��������ƿ������15mL10%Na2CO3��Һ��С�����10min��ʣ��ļ�Һ������������ˮ��Fe��ϴ�ɾ��������������������ã�
��1��Na2CO3��Һ�������dz�ȥ��м��������ۣ���ʣ��ļ�Һ�����ķ���ͨ������������
B��FeSO4���Ʊ�
�������������õ�Feм������ƿ�У�����25mL 3mol•L-1 H2SO4������ˮԡ�м�������������������Ϊֹ���ټ���1mL 3mol•L-1H2SO4�����ȹ��ˣ�����Һת�����������У�
��2�����ȹ��˵�Ŀ����FeSO4����Һ����������Ӧ����ʱ�ټ���1mL 3mol•L-1 H2SO4������pH��1���������Ƿ�ֹFeSO4������������笠����Ӻ���������ˮ�⣮
C����NH4��2SO4•FeSO4•6H2O���Ʊ�
��������ȡһ�����ģ�NH4��2SO4�������������ʵ����������У��������ȣ�������ȴ���õ���������淋ľ��壮���ˡ�ϴ�ӡ����������
��3���������ȣ�Ũ����Ũ����Һ�������־�ĤΪֹֹͣ���ȣ��Ƶõľ�����˺���Cϴ�ӣ�
A������ˮ B������ʳ��ˮ C����ˮ�Ҵ� D����Һ
��4�������������Һ������Ũ�ȵĴ�С��ϵc��SO42-����c��NH4+����c��Fe2+����c��H+����c��OH-��
��5����Ʒ�п��ܴ�������NH4Fe��SO4��2��д��NH4Fe��SO4��2��Һ�����Ba��OH��2��Һ��Ӧ�����ӷ���ʽNH4++Fe3++2SO42-+2Ba2++4OH-=2BaSO4��+Fe��OH��3��+NH3•H2O��
D����NH4��2SO4•FeSO4•6HO����;
��������什��̷��ȶ�����������ԭ�ζ������г���������Fe2+�ı���Һ����ȡ0.400g Cu2S��CuS�Ļ������������Һ����70.0mL 0.100mol•L-1 KMnO4��Һ������������Ӧ���£�
2MnO4-+Cu2S+8H+�T2Cu2++SO42-+2Mn2++4H2O
8MnO4-+5CuS+24H+�T5Cu2++5SO42-+8Mn2++12H2O
��Ӧ��ʣ���KMnO4ǡ����V mL 0.500mol•L-1 ��NH4��2SO4•FeSO4��Һ��ȫ��Ӧ��
��֪��
MnO4-+Fe2++H+-Fe3++Mn2++H2O��δ��ƽ����
��6��ʢKMnO4��Һ������ʽ�ζ��ܣ�V��ȡֵ��Χ3.3mL��20mL��mL��С�������1λ����
���� ��1������̼������ҺΪ������Һ���ܹ���ȥ���۷����������ܶȱȽϴ��Ҳ�����ˮ���ݴ��ж�ʣ��ļ�Һ�����ķ���
��2���¶�Խ�ߣ���������淋��ܽ��Խ������������pHֵ�ϴ�ʱ���ױ�������笠����Ӻ�����������ˮ�⣬�ݴ˷�����
��3������Һ�еõ���������淋ľ��壬����������Ũ�������½ᾧ�ķ����������о�Ĥ����ʱ����ֹͣ���ȣ���ֹĪ����ʧˮ����ϴ��ʱҪ��ȥ���������ʵ�ͬʱ��ֹ�����ܽ����ʧ��
��4�������������Һ���������Ӻ�笠����Ӷ���ˮ�⣬ʹ��Һ�����ԣ�
��5��NH4Fe��SO4��2��Һ�����Ba��OH��2��Һ��Ӧ���ɰ��������ᱵ�������������������ݵ���غ��Ԫ���غ���д���ӷ���ʽ��
��6��KMnO4��Һ����ǿ�����ԣ��������齺�ܣ����ݷ�Ӧ2MnO4-+Cu2S+8H+�T2Cu2++SO42-+2Mn2++4H2O��8MnO4-+5CuS+24H+�T5Cu2++5SO42-+8Mn2++12H2O�ɵù�ϵʽ2MnO4-��Cu2S��8MnO4-��5CuS������0.400g Cu2S��CuS�Ļ��������KMnO4��Һ�����ʵ����ķ�Χ��ȷ����NH4��2SO4•FeSO4�����ʵ���������ȷ����Һ�������
��� �⣺��1��Na2CO3��Һ��һ�ּ�����Һ�����ݶ�ԭ�ϳ���Ҫ�����Ƴ��ù����dz�ȥԭ���ϵ����ۣ������¶ȣ��ٽ���Na2CO3��ˮ�⣬��Һ������ǿ��ȥ����������ǿ�������ܶȱȽϴ��Ҳ�����ˮ�����Խ�ʣ��ļ�Һ�����ķ���ͨ������������
�ʴ�Ϊ����ȥ��м��������ۣ���������
��2���¶Ƚϸ������������������ܽ�Ƚϴ����Գ��ȹ��˵�Ŀ���Ƿ�ֹFeSO4����Һ������������pH��1���������Ƿ�ֹFeSO4������������笠����Ӻ���������ˮ�⣬
�ʴ�Ϊ��FeSO4����Һ����������ֹFeSO4������������笠����Ӻ���������ˮ�⣻
��3������Һ�еõ���������淋ľ��壬����������Ũ�������½ᾧ�ķ����������о�Ĥ����ʱ����ֹͣ���ȣ���ֹĪ����ʧˮ����ϴ��ʱҪ��ȥ���������ʵ�ͬʱ��ֹ�����ܽ����ʧ������Ҫ����ˮ�Ҵ�ϴ�ӣ���ѡC��
�ʴ�Ϊ��Ũ����Һ�������־�Ĥ��C��
��4�������������Һ���������Ӻ�笠����Ӷ���ˮ�⣬ʹ��Һ�����ԣ����ݣ�NH4��2SO4•FeSO4�Ļ�ѧʽ����Ԫ���غ㣬��֪��Һ������Ũ�ȵĴ�С��ϵ��c��SO42-����c��NH4+����c��Fe2+����c��H+����c��OH-����
�ʴ�Ϊ��c��SO42-����c��NH4+����c��Fe2+����c��H+����c��OH-����
��5��NH4Fe��SO4��2��Һ�����Ba��OH��2��Һ��Ӧ���ɰ��������ᱵ������������������Ӧ�����ӷ���ʽΪNH4++Fe3++2SO42-+2Ba2++4OH-=2BaSO4��+Fe��OH��3��+NH3•H2O��
�ʴ�Ϊ��NH4++Fe3++2SO42-+2Ba2++4OH-=2BaSO4��+Fe��OH��3��+NH3•H2O��
��6��KMnO4��Һ����ǿ�����ԣ��������齺�ܣ�����ʢKMnO4��Һ������ʽ�ζ��ܣ����ݷ�Ӧ2MnO4-+Cu2S+8H+�T2Cu2++SO42-+2Mn2++4H2O��8MnO4-+5CuS+24H+�T5Cu2++5SO42-+8Mn2++12H2O�ɵù�ϵʽ2MnO4-��Cu2S��8MnO4-��5CuS������0.400g��Ʒ��Ϊ Cu2S��������KMnO4��Һ�����ʵ���Ϊ$\frac{0.400��2}{160}$mol=0.005mol��ʣ���KMnO4�����ʵ���Ϊ0.007mol-0.005mol=0.002mol������0.400g��Ʒ��Ϊ CuS��������KMnO4�����ʵ���Ϊ$\frac{8��0.4}{5��96}$mol=0.00667mol��ʣ���KMnO4�����ʵ���Ϊ0.007mol-0.00667mol=0.00033mol����ʣ���KMnO4�����ʵ�����ΧΪ0.00033mol��0.002mol������MnO4-��5Fe2+��ȷ����NH4��2SO4•FeSO4�����ʵ����ķ�ΧΪ0.00165mol��0.01mol������ȷ����Һ�����V��ȡֵ��Χ��$\frac{0.00165}{0.5}$L��$\frac{0.01}{0.5}$L����Ϊ3.3mL��20mL��
�ʴ�Ϊ���3.3mL��20mL��
���� ��������������茶�����Ʊ�Ϊ���壬��Ҫ�����˳����ᴿ�Ļ�����������ʵ�鲽��ԭ����Ŀ�ķ������ۡ���װ�õķ������۵ȣ���ȷԭ���ǽ���ؼ���Ū���������Ļ�ѧ��Ӧ����Ԫ�ش�����̬�ı仯����Ŀ�Ѷ��еȣ�

| A�� | Al��Mg=Fe��Na | B�� | Al=Mg=Fe=Na | C�� | Na��Al��Mg��Fe | D�� | Al=Mg=Fe��Na |
| ������ | K+��Cu2+��Fe3+��Al3+��Fe2+ |
| ������ | Cl-��CO32-��NO3-��SO42-��SiO32- |
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��
��ȡ����ԭ��Һ������KSCN��Һ�����Ա仯��
����ȡԭ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䣮
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɣ�
���ƶϣ�
��1���ɢ��жϣ���Һ��һ�������е���������K+��Fe3+��д���ӷ��ţ���
��2�����м�����������������ɫ��������ӷ���ʽ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
��3�����������ú���ɫ����ͨ��ˮ�У��������ɫ���������Ļ�ѧ����ʽΪ3NO2+H2O=2HNO3+NO��
��4����ͬѧ����ȷ��ԭ��Һ��������������Fe2+��Mg2+����������Cl-��NO3-��SO42-��д���ӷ��ţ���
��5����ȡ50mLԭ��Һ������������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ��õ��Ĺ�������Ϊ0.6g��������һλС����
| A�� | ʹ�õζ���ʱ���ζ��ܱ����ô�װҺ��ϴ2��3�� | |
| B�� | ��ʽ�ζ��ܲ���ʢװ��Һ����ʽ�ζ��ܲ���ʢװ��Һ��ǿ��������Һ | |
| C�� | ʢװȷ��ȡ�Ĵ���Һ����ƿӦԤ���ô���Һ��ϴ | |
| D�� | �ζ��յ�ʱ�������������������Һ������װ�д���Һ�ĵζ����ٵ���һ�������е��� |
| A�� | CH2=CH-CH3 | B�� | CH2=CH-CH2-CH3 | ||
| C�� | CH3=CH-CH2-CH=CH2 | D�� | CH2=CH-CH2-CH2 |
| A�� | AB2 | B�� | A2B | C�� | AB | D�� | A2B3 |
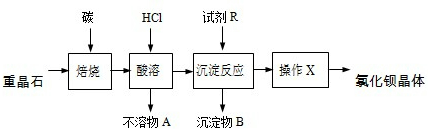
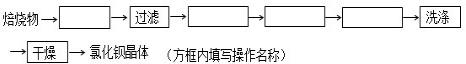


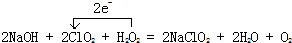 ������װ���е��¶Ȳ��ܹ��ߣ���ԭ���ǣ���ֹH2O2�ķֽ⣮
������װ���е��¶Ȳ��ܹ��ߣ���ԭ���ǣ���ֹH2O2�ķֽ⣮