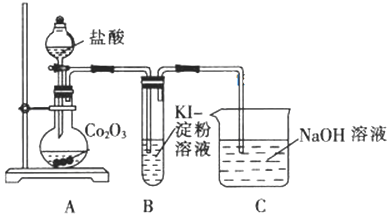
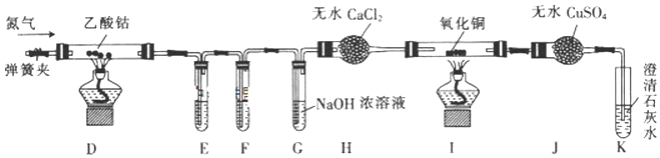
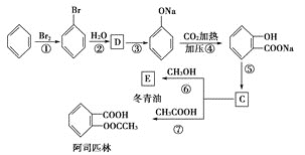
��Ŀ����
����Ŀ����KMnO4�����ױ��Ʊ������ᡣʵ�鷽�������ױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�����ˣ������б�����أ�C6H5COOK���ͼױ�����Һ���������̷��뱽���Ტ����δ��Ӧ�ļױ���

����˵����ȷ���ǣ� ��
A.��ɫҺ��A�DZ����ᣬ��ɫ����B�Ǽױ�
B.�����������Ϊ������II������Ϊ��Һ
C.Ϊ�˵õ�����İ�ɫ����B����ȴ�ᾧʱ�¶�Խ��Խ��
D.����Ũ�����ữ��Ŀ����Ϊ�˽��������ת��Ϊ����
���𰸡�D
��������
�����̵�Ŀ���ǴӺ��б�����غͼױ�����Һ�з���������Ტ���ռױ���Χ�����Ŀ�ģ�����������Ϊ����Һ����Һ������л���Һ��ˮ��Һ���л���Һ����Ҫ�ɷ��Ǽױ�����Na2SO4�����ˮ�����˺�����ʹ���������������е��ε����ʷ��룬ʵ�ֱ��Ļ��գ�ˮ��Һ��Ũ�����ữ��ʹ�������ת���ɱ����ᣬ����ȴ�ᾧ�����˴�ˮ��Һ�з����������ñ����ᡣ
A. �ӷ�����֪����Һ����������ˮ�����ܣ�����Һ����õ����л���Һ��Ҫ�ɷ��Ǽױ�����Na2SO4�����ˮ�����˺������õ���ɫҺ��A�Ǽױ���Aѡ�����
B. �������Ŀ���Ƿ��벻���ܵ��л���Һ��ˮ��Һ��ӦΪ��Һ������II�ǽ��ױ����������е����ʷ��룬��ȷ�IJ���Ϊ����Bѡ�����
C. ��ȴ�ᾧ��Ŀ����ʹ�������ˮ��Һ�з���������¶ȹ��ͽ�ʹ��Һ�е�����Ҳ������ʹ���������Cѡ�����
D. Ϊ��ˮ��Һ�л�ñ����ᣬ�ɼ���Ũ�����ữ�����������ת��Ϊ�����ᣬ�������ڱ������ܽ�ȱȱ�����صĵͣ������ں������룬Dѡ����ȷ��
��ѡD��