��Ŀ����
�����������Ʊ�ʵ��
Ҫ������Ʊ�����������ʵ�鷽��
���ṩ�Լ��ǣ��������ۼ����ʣ������̼�ۣ������ۼ�10 mL 3 mlo/L H2SO4
ʵ��Ŀ�ģ�ѡ���������������Ʊ���;������һ���˽��������ķ���
��Ӧԭ�����������ᷴӦ��ȡ��������
ʵ����Ʒ��
ʵ�鲽�裺
ʵ�������¼�����������
˼�������ۣ�
��1��Ϊʲôѡ����3mol/L H2SO4��Һ��������Ũ�ȸ���һ��ģ�����ʾ���ɿ������������ڳ����µ��ܽ�ȣ�
��2��ΪʲôҪ���ȹ��ˣ�
����ʾ��Ҫ�������������ڲ�ͬ�¶��µ��ܽ�ȣ�
��3������Ϊ��ʵ������еḶ́�Һ�����м��֣������ʺ�����Щ�����
Ҫ������Ʊ�����������ʵ�鷽��
���ṩ�Լ��ǣ��������ۼ����ʣ������̼�ۣ������ۼ�10 mL 3 mlo/L H2SO4
ʵ��Ŀ�ģ�ѡ���������������Ʊ���;������һ���˽��������ķ���
��Ӧԭ�����������ᷴӦ��ȡ��������
ʵ����Ʒ��
ʵ�鲽�裺
ʵ�������¼�����������
˼�������ۣ�
��1��Ϊʲôѡ����3mol/L H2SO4��Һ��������Ũ�ȸ���һ��ģ�����ʾ���ɿ������������ڳ����µ��ܽ�ȣ�
��2��ΪʲôҪ���ȹ��ˣ�
����ʾ��Ҫ�������������ڲ�ͬ�¶��µ��ܽ�ȣ�
��3������Ϊ��ʵ������еḶ́�Һ�����м��֣������ʺ�����Щ�����
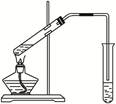
ʵ����Ʒ��50mL�ձ�2ֻ������������Ͳ������©�����ƾ��ơ���ͷ�ιܡ������ֽ2�š�����̨����Ȧ��ʯ������������ƽ�����ۡ�3mol/L H2SO4
ʵ�鲽�裺
��1����������ϴ����������ƽ��ȡ�������ʵ�����2g������50mL�ձ�֮�У�����10mL���ҵ�ϡ����������Һ�����貢�������У����䡢�������㵹ȥ�ϲ���Һ���ټ�������ˮ20mL���ң����衢�������㵹ȥ�ϲ���Һ��������Σ�ֱ����Һ�����ԡ�
��2�����������������������ձ��м���10mL 3mol/L H2SO4��Һ���������������ַ�Ӧ�����þƾ������ȣ���ַ�ӦԼ20���ӡ�
��3�����ȹ��ˣ���������Һ���ȹ��ˣ�����Ҫ�졣Ϊʲô�������õ����������Ĺ�������Һ����ȴ�����£��õ����������ľ��塣
��4����ȡ���壺������3���û������һ�ι��ˣ���������ˮϴ��2��3�Σ��ɵõ����������ľ��塣
��5��ʵ�����������ɡ�
ʵ�������¼�������������1�������Ͽɻ�þ����������2��ʵ�ʻ�õľ����������3����������ԭ�����
����ʵ����̵����ա�ʵ����Ʒ��50mL�ձ�2ֻ������������Ͳ������©�����ƾ��ơ���ͷ�ιܡ������ֽ2�š�����̨����Ȧ��ʯ������������ƽ�����ۡ�3mol/L H2SO4
ʵ�鲽�裺
��1����������ϴ����������ƽ��ȡ�������ʵ�����2g������50mL�ձ�֮�У�����10mL���ҵ�ϡ����������Һ�����貢�������У����䡢�������㵹ȥ�ϲ���Һ���ټ�������ˮ20mL���ң����衢�������㵹ȥ�ϲ���Һ��������Σ�ֱ����Һ�����ԡ�
��2�����������������������ձ��м���10mL 3mol/L H2SO4��Һ���������������ַ�Ӧ�����þƾ������ȣ���ַ�ӦԼ20���ӡ�
��3�����ȹ��ˣ���������Һ���ȹ��ˣ�����Ҫ�졣Ϊʲô�������õ����������Ĺ�������Һ����ȴ�����£��õ����������ľ��塣
��4����ȡ���壺������3���û������һ�ι��ˣ���������ˮϴ��2��3�Σ��ɵõ����������ľ��塣
��5��ʵ�����������ɡ�
ʵ�������¼�������������1�������Ͽɻ�þ����������2��ʵ�ʻ�õľ����������3����������ԭ�����
ʵ�鲽�裺
��1����������ϴ����������ƽ��ȡ�������ʵ�����2g������50mL�ձ�֮�У�����10mL���ҵ�ϡ����������Һ�����貢�������У����䡢�������㵹ȥ�ϲ���Һ���ټ�������ˮ20mL���ң����衢�������㵹ȥ�ϲ���Һ��������Σ�ֱ����Һ�����ԡ�
��2�����������������������ձ��м���10mL 3mol/L H2SO4��Һ���������������ַ�Ӧ�����þƾ������ȣ���ַ�ӦԼ20���ӡ�
��3�����ȹ��ˣ���������Һ���ȹ��ˣ�����Ҫ�졣Ϊʲô�������õ����������Ĺ�������Һ����ȴ�����£��õ����������ľ��塣
��4����ȡ���壺������3���û������һ�ι��ˣ���������ˮϴ��2��3�Σ��ɵõ����������ľ��塣
��5��ʵ�����������ɡ�
ʵ�������¼�������������1�������Ͽɻ�þ����������2��ʵ�ʻ�õľ����������3����������ԭ�����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
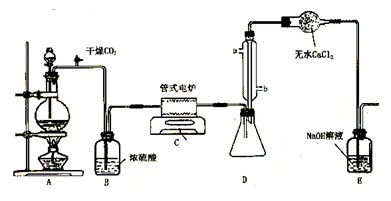
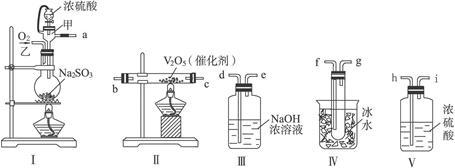
 ������ͼ��ʾ(װ�ÿ��ظ�ʹ��)���ش��������⣺
������ͼ��ʾ(װ�ÿ��ظ�ʹ��)���ش��������⣺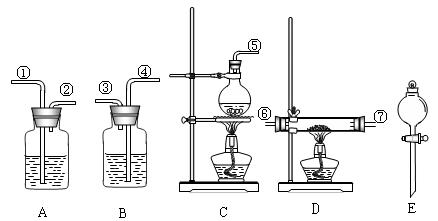
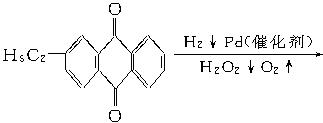
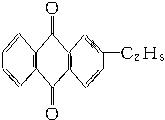

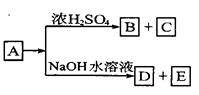
 +CaCl2+H2O�����Ʊ�
+CaCl2+H2O�����Ʊ�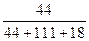 ��100%������ɫ��ѧ�����У�ԭ�ӵ�������Ϊ100%�Ĺ��ճ�����״̬�ġ���ɫ���ա��������ṩ������H2O2�ķ������ɷ��Ϊ����״̬�ġ���ɫ���ա����������ɡ�
��100%������ɫ��ѧ�����У�ԭ�ӵ�������Ϊ100%�Ĺ��ճ�����״̬�ġ���ɫ���ա��������ṩ������H2O2�ķ������ɷ��Ϊ����״̬�ġ���ɫ���ա����������ɡ�