��Ŀ����
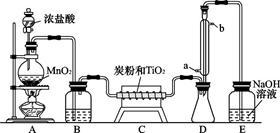
(9��) ���Ȼ�������ɫҺ�壬�е�Ϊ136�档������ˮ�⣬��������ˮ���������������̡���TiCl4+H2O=TiOCl2+2HCl��������650��~850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ʵ�����Ʊ�TiCl4�ķ�Ӧװ�ã�����Ҫ�����������£�
�����Ӻ�����װ�ã���ͨCl2ǰ��ͨ��CO2���岢����һ��ʱ�䣻
�ڵ���ƿ��TiCl4������������ʱ��ֹͣ���ȣ��Ӳ���и�ͨCO2����ֱ����¯�еĴɹ���ȴΪֹ��
�۽�TiO2��̿�ۻ�Ͼ��Ⱥ�װ���ʽ��¯�У�
�� ����¯���µ�800�棬һ��ʱ����ͨCl2��ͬʱ����������ͨ����ˮ��
�Իش��������⣺
��1����ȷ�IJ���˳��Ϊ������ţ� ��
��2��װ��A�еķ�Ӧ�����ӷ���ʽΪ ��
��3��Cװ���еķ�Ӧ����ʽΪ�� ��
��4�������ٵ�Ŀ���� ��
��5��װ��D�������ܽ�ˮ�ڵ�λ���ǣ���a��b�� ��װ��E�������� ��
�����Ӻ�����װ�ã���ͨCl2ǰ��ͨ��CO2���岢����һ��ʱ�䣻
�ڵ���ƿ��TiCl4������������ʱ��ֹͣ���ȣ��Ӳ���и�ͨCO2����ֱ����¯�еĴɹ���ȴΪֹ��
�۽�TiO2��̿�ۻ�Ͼ��Ⱥ�װ���ʽ��¯�У�
�� ����¯���µ�800�棬һ��ʱ����ͨCl2��ͬʱ����������ͨ����ˮ��

�Իش��������⣺
��1����ȷ�IJ���˳��Ϊ������ţ� ��
��2��װ��A�еķ�Ӧ�����ӷ���ʽΪ ��
��3��Cװ���еķ�Ӧ����ʽΪ�� ��
��4�������ٵ�Ŀ���� ��
��5��װ��D�������ܽ�ˮ�ڵ�λ���ǣ���a��b�� ��װ��E�������� ��
��1�� �ۢ٢ܢ� ��2�֣� ��2��MnO2+4H+ +2 Cl��
 Mn2+ + Cl2��+2H2O ��1�֣�
Mn2+ + Cl2��+2H2O ��1�֣���3��TiO2+2Cl2+2C
 TiCl4+2CO��2�֣�
TiCl4+2CO��2�֣���4���ž�װ���ڵĿ���������TiCl4��������ˮ��������ˮ�⣨2�֣�
��5�� b ��1�֣������ն����Cl2���ӷ���HCl��������Ⱦ������1�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
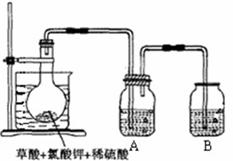
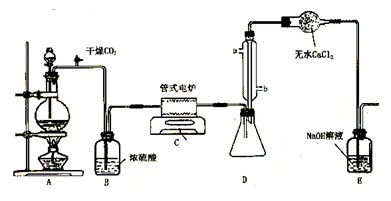
 TiOCl2+2HCl��������650��850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ʵ�����Ʊ�TiCl4�IJ���װ�á�
TiOCl2+2HCl��������650��850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ʵ�����Ʊ�TiCl4�IJ���װ�á�