��Ŀ����
6����һ�����Ϊ5L������ܱ������м���1.25molCaCO3��������ӦCaCO3��s��?CaO ��s��+CO2��g�������ƽ��ʱ������̼�����ʵ���Ũ�����¶ȵı仯��ϵ��ͼ1��ʾ���밴Ҫ��ش��������⣺
��1���÷�Ӧ����ӦΪ���ȷ�Ӧ��������š������¶�ΪT5��ʱ���÷�Ӧ��ʱ40s�ﵽƽ�⣬��T5��ʱ����ʽ����÷�Ӧ�ķ�Ӧ����Ϊ0.005mol/��L•s����
��2��T2��ʱ���÷�Ӧ�Ѿ��ﵽƽ�⣬���д�ʩ����ʹ�÷�Ӧ��ƽ�ⳣ��Kֵ������d��ѡ���ţ���
a����Сѹǿ b������CaO���� c������CaCO3 d�������¶�
��3������ͼ2�л���ƽ�ⳣ��K���¶ȵı仯���ߣ�
��4����T5���£�ά���¶Ⱥ�����������䣬������ƽ����ϵ���ٳ���1molN2�������ƽ��ʱ�����е�CaCO3������Ϊ25g��
���� ��1����ͼ��֪���¶�Խ�ߣ�������̼�����ʵ���Ũ��Խ��˵���˷�ӦΪ���ȷ�Ӧ��T5��ʱ������̼�����ʵ���Ũ��Ϊ0.2mol/L���ݴ˼��㷴Ӧ���ʼ��ɣ�
��2��CaCO3��s��?CaO ��s��+CO2��g�����˷�Ӧ�ص�Ϊ���ȷ�Ӧ��K���¶ȵĺ������ݴ˽�ɣ�
��3���¶�Խ�ߣ�ƽ�ⳣ��Խ�ݴ˽�ɣ�
��4��������䣬���뵪�����Ի�ѧ��Ӧƽ����Ӱ�죬�ݴ˽�ɣ�
��� �⣺��1���¶�Խ�ߣ�������̼�����ʵ���Ũ��Խ�˷�ӦΪ���ȷ�Ӧ��T5��ʱ������̼�����ʵ���Ũ��Ϊ0.2mol/L��v��CO2��=$\frac{c��C{O}_{2}��}{��t}=\frac{0.2mol/L}{40s}$=0.005mol/��L•s�����ʴ�Ϊ������0.005mol/��L•s����
��2��CaCO3��s��?CaO ��s��+CO2��g�����˷�Ӧ�ص�Ϊ���ȵķ�Ӧ��Kֻ���¶��йأ������¶ȣ�ƽ�����ƣ�Kֵ����ѡd��
��3���¶�Խ�ߣ�ƽ�ⳣ��Խ����ƽ�ⳣ����Ϊ������̼��Ũ�ȣ���ͼ������Ϊ��������ͼ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��ά���¶Ⱥ�����������䣬������ƽ����ϵ���ٳ���1molN2���Դ�ƽ����Ӱ�죬�����ɶ�����̼��Ũ����ȻΪ0.2mol/L�����Ϊ5L����ô���ʵ���Ϊ5L��0.2mol/L=1mol�����ݷ�Ӧ��CaCO3��s��?CaO ��s��+CO2��g����
100 44
x 44g����x=100g����ôʣ��̼�������Ϊ125g-100g=25g���ʴ�Ϊ��25��
���� ������Ҫ������ǻ�ѧ��Ӧ���ʵļ��㡢���淴Ӧ�ص㣨���ȡ����ȣ����жϣ�ƽ�ⳣ����Ӱ�����صȣ��ۺ��Խ�ǿ�������ѶȲ���

| A�� | n��Fe����n��S��������������44 g | B�� | n��Fe����n��S������������44 g | ||
| C�� | n��Fe��=n��S��������������44 g | D�� | n��Fe����n��S��������������44 g |
| A�� | ��������Һ�м�������İ�ˮ��Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | |
| B�� | ������Ͷ��ˮ�У�Na+H2O�TNa++OH-+H2�� | |
| C�� | ����Ƭ�������NaOH��Һ�У�2Al+2OH-+2H2O�T2AlO2-+3H2�� | |
| D�� | ����������NaOH��Һ��Al2O3+2OH-�T2AlO2-+H2O |
| A�� | ��̬ԭ�Ӻ���δ�ɶԵ������ĵڶ�����Ԫ�� | |
| B�� | λ�����ڱ��е������ڵڢ�A���Ԫ�� | |
| C�� | ��̬ԭ��������Ӳ��Ų�Ϊ2s22p6��Ԫ�� | |
| D�� | ��̬ԭ��������Ӳ��Ų�Ϊ3s23p5��Ԫ�� |
| A�� | ��Щ�������ɷ���ֱ�ӹ��ɵģ���ѧʽ��ȷ��ʾ�����ʷ�����ɣ�����ף�P4����������̼��CO2�����Ȼ�泥�NH4Cl���� | |
| B�� | C2H2��BeCl2�����е�����ԭ���ӻ������������ͬ | |
| C�� | ��1molSiO2����������2molSi-O�� | |
| D�� | NC13������N-C1��������CCl4������C-C1�������� |

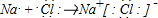
 ��������������Ӧ��
��������������Ӧ�� ����Fe��OH��2���ױ�����������ʵ����������������Һ���ռӦ�Ƶð�ɫ������Fe��OH��2������������ͼ��ʾʵ��װ�ÿ��Ƶô�����Fe��OH��2�������������Ϸֱ�Ϊʯī������
����Fe��OH��2���ױ�����������ʵ����������������Һ���ռӦ�Ƶð�ɫ������Fe��OH��2������������ͼ��ʾʵ��װ�ÿ��Ƶô�����Fe��OH��2�������������Ϸֱ�Ϊʯī������ ��
��