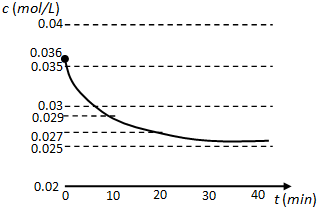
��Ŀ����
3��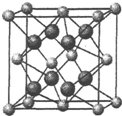 ������Ȼ���г���CaF2����ʽ���ڣ�
������Ȼ���г���CaF2����ʽ���ڣ���1����ԭ�ӵļ۵����Ų�ʽΪ2s22p5��HF���������������ˮ��������ΪHF�Ǽ��Է����⣬����ΪH2O��HF�γɷ��Ӽ������
��2��F2ͨ��ϡNaOH��Һ�п�����OF2��OF2���ӹ���ΪV�Σ�������ԭ�ӵ��ӻ���ʽΪsp3��F2������±�ص��ʷ�Ӧ�����γ�±�ػ��������ClF3��BrF3�ȣ�ClF3�ķе��BrF3�ĵ� ����ߡ��͡�����
��3�������й�CaF2�ı�����ȷ����bd��
a��Ca2+��F-������ھ�����������
b��F-�����Ӱ뾶С��Cl-����CaF2���۵����CaCl2
c���������ӱ�Ϊ2��1�����ʣ�����CaF2���幹����ͬ
d��CaF2�еĻ�ѧ��Ϊ���Ӽ������CaF2������״̬���ܵ���
��4��CaF2������ˮ���������ں�Al3+����Һ�У�ԭ����3CaF2+Al3+=3Ca2++AlF63-�������ӷ���ʽ��ʾ���� ��֪AlF63-����Һ�п��ȶ����ڣ�
��5��CaF2�����ṹ��ͼ��CaF2�����У�F-����λ����4����һ��Ca2+�Ⱦ����������Ca2+��12������֪CaF2�����ܶ�Ϊdg/cm3����F-��F-����̾���Ϊ$\root{3}{\frac{39}{d{N}_{A}}}$cm���ú�d�Ĵ���ʽ��ʾ��
���� ��1��F��ԭ�Ӻ���9�����ӣ��������Ų����������7�����ӣ�HF��ˮ���Ӽ����γ������
��2�����ݼ۲���ӶԻ������۷������ȼ���۲���Ӷ��������ж�����ԭ�ӵ��ӻ����ͣ������ӹ��ͣ�±�ػ��������Է�������Խ����е�Խ�ߣ�
��3��a���������Ӽ���ھ��������;����������
b�����Ӿ�����۵�������������ɡ����Ӱ뾶�йأ�
c������Ľṹ���ɱȡ��뾶���йأ�
d�����ӻ�����������ʱ�ܷ������룮
��4��F-��Al3+���γɺ��ѵ����������AlF63-��
��5��CaF2������������Ӿ�������ķ�������8�������ݾ����ṹ����ϸ����ӵ�λ���жϣ������и�����λ�ھ�����8�������6�����ϣ�$\frac{1}{8}$��8+$\frac{1}{6}$��2=4��8��������λ�ھ����ڲ�����һ�������к���4��CaF2���������V=$\frac{\frac{4M}{{N}_{A}}}{��}$���Ѿ�������Ϊ8��С�����壬��ÿ��С�������к���һ�������ӣ�F-��F-����̾������С��������ⳤ��
��� �⣺��1��F��ԭ�Ӻ���9�����ӣ��������Ų����������7�����ӣ���F�ļ۵����Ų�ʽΪ2s22p5��H2O��HF�γɷ��Ӽ�����������ܣ�
�ʴ�Ϊ��2s22p5��H2O��HF�γɷ��Ӽ������
��2��OF2������Oԭ�ӵļ۲���Ӷ���=2+$\frac{1}{2}$��6-2��1��=4����Oԭ�ӵ��ӻ�����Ϊsp3�ӻ�������2���µ��Ӷԣ����Է��ӵĿռ乹��ΪV�Σ�±�ػ��������Է�������Խ����е�Խ�ߣ�����ClF3�ķе��BrF3�ĵͣ�
�ʴ�Ϊ��V�Σ�sp3���ͣ�
��3��a���������Ӽ���ھ��������;��������Ca2+��F-����ھ����������ã������ھ����������a����
b�����Ӿ�����۵�������������ɡ����Ӱ뾶�йأ����Ӱ뾶ԽС�����Ӿ�����۵�Խ�ߣ�����CaF2���۵����CaCl2����b��ȷ��
c������Ľṹ���ɱȡ��뾶���йأ��������ӱ�Ϊ2��1�����ʣ���CaF2����ĵ�ɱ���ͬ�����뾶�����ϴ����幹�Ͳ���ͬ����c����
d��CaF2�еĻ�ѧ��Ϊ���Ӽ������ӻ�����������ʱ�ܷ������룬���������ƶ������ӣ��ܵ��磬���CaF2������״̬���ܵ��磬��b��ȷ��
�ʴ�Ϊ��bd��
��4��CaF2������ˮ���������ں�Al3+����Һ�У���Ϊ����Һ��F-��Al3+���γɺ��ѵ����������AlF63-��ʹCaF2���ܽ�ƽ�����ƣ��䷴Ӧ�����ӷ���ʽΪ��3CaF2+Al3+=3Ca2++AlF63-��
�ʴ�Ϊ��3CaF2+Al3+=3Ca2++AlF63-��
��5��CaF2������������Ӿ�������ķ�������8������������λ��Ϊ8��ͼ����ÿ����������Ca2+��������ҵȾ����Ca2+������Ϊ3����ͨ��ÿ��Ca2+���γ�8��������ÿ��Ca2+����2�Σ�������Ca2+��������ҵȾ����Ca2+������Ϊ��8��3����2=12����
�����и�����λ�ھ�����8�������6�����ϣ�$\frac{1}{8}$��8+$\frac{1}{6}$��2=4��8��������λ�ھ����ڲ�����һ�������к���4��CaF2���������V=$\frac{\frac{4M}{{N}_{A}}}{��}$���Ѿ�������Ϊ8��С�����壬��ÿ��С�������к���һ�������ӣ�С����������Ϊ$\frac{\frac{4M}{{N}_{A}}}{��}$��$\frac{1}{8}$=$\frac{39}{��{N}_{A}}$��F-��F-����̾������С��������ⳤ����F-��F-����̾���Ϊ$\root{3}{\frac{39}{��{N}_{A}}}$=$\root{3}{\frac{39}{d{N}_{A}}}$��
�ʴ�Ϊ��4��12��$\root{3}{\frac{39}{d{N}_{A}}}$��
���� ���⿼�������ʽṹ�����ʣ���Ŀ�漰�����۷е�ıȽϡ���ѧ���������ܽ�ƽ�⡢�ӻ����۵�Ӧ�á������ļ���ȣ���Ŀ�漰��֪ʶ��϶࣬�����ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | AlCl3 | B�� | KHCO3 | C�� | Fe2��SO4��3 | D�� | NH4HCO3 |
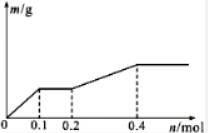 ��Pt�缫��⺬��Ag+��Cu2+��X3+��0.1mol����Һ�����������������ʵ�����m��g�����·��ͨ�����ӵ����ʵ���n��mol���Ĺ�ϵ��ͼ��ʾ��������������ǿ�����ж���ȷ���ǣ�������
��Pt�缫��⺬��Ag+��Cu2+��X3+��0.1mol����Һ�����������������ʵ�����m��g�����·��ͨ�����ӵ����ʵ���n��mol���Ĺ�ϵ��ͼ��ʾ��������������ǿ�����ж���ȷ���ǣ�������| A�� | Ag+��X3+��Cu2+��H+��X2+ | B�� | Ag+��Cu2+��X3+��H+��X2+ | ||
| C�� | Cu2+��X3+��Ag+��X2+��H+ | D�� | Cu2+��Ag+��X3+��H+��X2+ |
| A�� | ��¯�г�����ˮ���ݿ�����ϡ�����ܽ�ȥ�� | |
| B�� | ����FeCl3������Һ�Ʊ�Fe��OH��3�ٽ���Fe3+ˮ�� | |
| C�� | ��ˮ�м���������¶���ʹˮ�����ӻ���С��ˮ�ĵ���ƽ�ⶼ�����ƶ� | |
| D�� | ��Ӧ2A��g��+B��g���T3C��s��+D��g����һ�����������Է����У�˵���÷�Ӧ�ġ�H��0 |
| A�� | H2O2�ĵ���ʽ��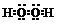 | |
| B�� | �Ҵ��Ľṹʽ��C2H6O | |
| C�� | ��ԭ�ӵĽṹʾ��ͼ�� | |
| D�� | FeSO4 �ĵ��뷽��ʽ��FeSO4=Fe3++SO42�� |
| A�� | ��Ӧ�����ɵ���ֻ��Fe��NO3��2 | B�� | ��Ӧ�����ɵ���ֻ��Fe��NO3��3 | ||
| C�� | ��Ӧ�����ɵ���ΪFe��NO3��2��Fe��NO3��3 | D�� | ��������������п��� |
| Ԫ�ط��ţ�Cs �������ƣ�� Ӣ�����ƣ�Cesium ԭ��������55 ���ԭ��������132.9 ��������Ų���2��8��18��18��8��1 |
| A�� | ����Ϊͬλ�� | |
| B�� | ����ԭ�Ӻ������������3 | |
| C�� | �����Ȼ���Ļ�ѧʽ�����Ա�ʾΪCsCl2 | |
| D�� | CsԪ��λ�����ڱ��������� |