��Ŀ����
��A�����������(Se)����(Te)��Ԫ���ڻ������г����ֳ����ֻ��ϼۣ�����A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
��1����Ԫ�����γɷ�����������д��һ����CO2�ȵ��ӵĻ����� ��
��2����Na2O��SiO2��P2O5���������ﰴ�۷е��ɸߵ���˳������ ��
��3��ԭ�ӵĵ�һ��������ָ��̬�����Ի�̬ԭ��ʧȥһ������ת��Ϊ��̬��̬����������Ҫ�����������O��S��Seԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ ��
��4��Seԭ�ӻ�̬������ӵ��Ų�ʽΪ ��H2Se�ķе㣺-41.1�� ��H2S�ķе㣺-60.4�棬�������߷е�������Ҫԭ���� ��
��5��SO32-��������ԭ�ӵ��ӻ���ʽ ,�����ӵ����幹��Ϊ ��
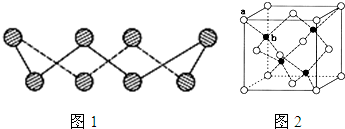
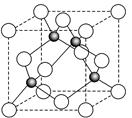
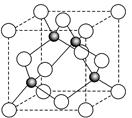
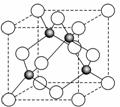
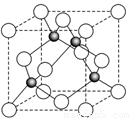
��6��ij����Ԫ��A���������������������������ϡ������������������ľ����ṹ����ͼ��ʾ��
���������Ļ�ѧʽΪ ��
��12�֣�[��4������5������ÿ��1�֣�����ÿ��2��]
(1)CS2 ��N2O (2)SiO2��Na2O��P2O5 (3) O��S��Se
(4)1s22s22p63s23p63d104s24p4 ��[Ar]3d104s24p4 H2Se����֮���������ǿ��H2S
(5) sp3�������� (6) AO
��������
�����������1��ԭ�����ͼ۵������ֱ���ȵĻ�Ϊ�ȵ����壬CO2����3��ԭ�ӡ�4��6��2��16���۵�������������CO2�ȵ��ӵĻ�������CS2 ��N2O��
��2��Na2O��SiO2��P2O5�����������γɵľ������ͷֱ������Ӿ��塢ԭ�Ӿ���ͷ��Ӿ��壬�������������ﰴ�۷е��ɸߵ���˳��������SiO2��Na2O��P2O5��
��3���ǽ�����Խǿ����һ������Խ����Ԫ�������ɿ�֪��ͬ����Ԫ�����϶��·ǽ�������������O��S��Se����Ԫ�صķǽ�����ǿ��˳����O��S��Se����������ԭ�ӵĵ�һ�������ɴ�С��˳��ΪO��S��Se��
��4��SeԪ�ص�ԭ��������34�����Ը��ݹ���ԭ����֪��Seԭ�ӻ�̬������ӵ��Ų�ʽΪ1s22s22p63s23p63d104s24p4 ��[Ar]3d104s24p4������H2Se��H2S�����γɵľ������;��Ƿ��Ӿ��壬��H2Se����֮���������ǿ��H2S������H2Se�ķ��ӷе����H2S�ķе㡣
��5�����ݼ۲���ӶԻ������ۿ�֪��SO32-��������ԭ�Ӻ��еŶԵ��Ӷ�������6��2��3��2����2��1�����Ը����ӵĿռ乹���������Σ�����ԭ�ӵ��ӻ���ʽ��sp3��
��6�����ݾ����ṹ�������ھ�̯����֪�������к��еİ��������8�� ��6��
��6��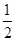 ��4����������ȫ���ھ����ڣ��������4�������Ը�������Ļ�ѧʽ��AO��
��4����������ȫ���ھ����ڣ��������4�������Ը�������Ļ�ѧʽ��AO��
���㣺����ȵ����塢����е�Ƚ��Լ������廯ѧʽȷ������һ�����ܡ���������Ų����ռ乹�͡��ӻ�������͵��жϵ�

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�