��Ŀ����
��12�֣���A�����������(Se)����(Te)��Ԫ���ڻ������г����ֳ����ֻ��ϼۣ�����A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
��1����Ԫ�����γɷ�����������д��һ����CO2�ȵ��ӵĻ����� ��
��2����Na2O�� SiO2��P2O5���������ﰴ�۷е��ɸߵ���˳������ ��
��3��ԭ�ӵĵ�һ��������ָ��̬�����Ի�̬ԭ��ʧȥһ������ת��Ϊ��̬��̬����������Ҫ�����������O��S��Seԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ ��
��4��Seԭ�ӻ�̬������ӵ��Ų�ʽΪ ��H2Se�ķе㣺��41.1�棬H2S�ķе㣺-60.4���������߷е�������Ҫԭ���� ��
��5��SO32-��������ԭ�ӵ��ӻ���ʽ ,�����ӵ����幹��Ϊ ��
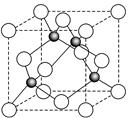
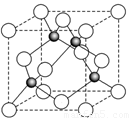
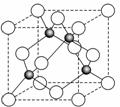
��6��ij����Ԫ��A���������������������������ϡ������������������ľ����ṹ����ͼ��ʾ�����������Ļ�ѧʽΪ ��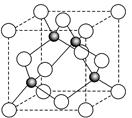
21����12�֣�
(1)CS2��N2O (2)SiO2>Na2O>P2O5 (3) O��S��Se
(4) [Ar]3d104s24p4 H2Se����֮���������ǿ��H2S
(5) sp3�������� (6) AO ��ÿС��2�֣�
��������
���������1��CO2����22�����ӣ�����ȵ��ӵĻ�������CS2��N2O
2��Na2O�� SiO2��P2O5����������ֱ�Ϊ���Ӿ��塢ԭ�Ӿ��塢���Ӿ��壬�������۷е�Ӧ��SiO2>Na2O>P2O5
3��O��S��Seԭ�Ӱ뾶����������ԭ�Ӻ˶Ժ�����ӵ���������С����һ�������ɴ�С��˳��ΪO��S��Se
4��SeΪ34��ԭ�ӣ�ԭ�ӻ�̬������ӵ��Ų�ʽΪ[Ar]3d104s24p4������H2Se����֮���������ǿ��H2S�����Բų������������
5��SO32-��������ԭ�ӵ��ӻ���ʽsp3�������ӵ����幹��Ϊ������
6ͼ�а�ɫС����4������ɫС��Ҳ��4�������Ը�������Ļ�ѧʽΪAO
���㣺�������۷е�ıȽϡ�ԭ�Ӻ�����ӵ��Ų��������Ľṹ����
�����������������֪ʶ��϶࣬�������������֪ʶ��Ϊ������֪ʶ�������ѶȲ��ؼ������ھ����ṹ�ķ�����ԭ�Ӻ�������Ų�ʽ����д��ƽʱӦ����ۺͼ�ǿ�ⷽ���ѵ����

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�