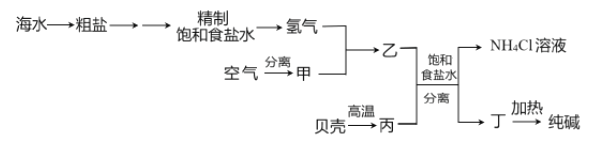
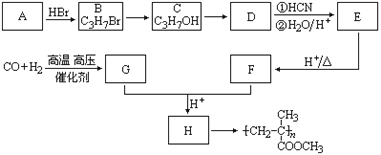
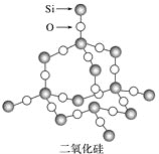
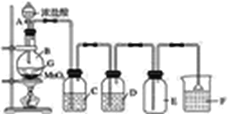
��Ŀ����
����Ŀ��Ϊ̽����ˮ�к��еIJ������Ӽ�ijЩ���ӵ����ʣ�ij��ѧ��ȤС����������ʵ�飺
��1���۲���ˮ��ɫ��������ˮ�ʻ���ɫ��֤����ˮ�к��е�������________��
��2������ˮ�е���̼������Һ�����������ɣ�˵����ˮ�к��е�������________��
��3��ʢ����ˮ���Թ��е���ɫ������ɫ��˵����ˮ�к��е�������________��
��4������ɫʯ����Һ����������ˮ�У���Һ�Ժ�ɫ�������õ�����________����һ�������Һ����ɫ��ȥ�������õ�����________��
��5������ˮ�еμ���������Һ�а�ɫ�������ɣ�֤����ˮ�к��е�������________��
��6����ˮ�����պ���ɫ����ʧ���ų���������________����Һ������________�����ǿ�������䡱����������
��7��������ͨ��ˮ�У�������Һ�о��������Եĺ�������________��
���𰸡�![]()
![]()
![]()
![]()
![]()
![]()
![]() ��ǿ
��ǿ ![]() ��
��![]() ��
��![]()
��������
��1������ˮ�У�ֻ��Cl2�ʻ���ɫ��������������ɫ����Ϊ��Cl2��
��2����ˮ�е�H+������̼������Һ���������塣��Ϊ��H+��
��3������ˮ�У�HClO����Ư���ԣ���ʹ��ɫ������ɫ����Ϊ��HClO��
��4������ɫʯ����Һ����������ˮ�У����Ժ�ɫ�����ֳ����ԣ������õ�����H+������ɫ�����ֳ������ԣ������õ�����HClO����Ϊ��H+��HClO��
��5����ˮ�е�Cl-��������������Ӧ�����ɰ�ɫ������֤����ˮ�к��е�������Cl-����Ϊ��Cl-��
��6����ˮ�е�HClO�����ֽ⣬������Ӧ2HClO![]() 2HCl+O2�����ų���������O2����Һ��������ǿ����Ϊ��O2����ǿ��
2HCl+O2�����ų���������O2����Һ��������ǿ����Ϊ��O2����ǿ��
��7��������ͨ��ˮ�У�������ӦCl2+H2O![]() HCl+HClO��HClO
HCl+HClO��HClO![]() H++ClO-��������Һ�о��������Եĺ�������Cl2��HClO��ClO-����Ϊ��Cl2��HClO��ClO-��
H++ClO-��������Һ�о��������Եĺ�������Cl2��HClO��ClO-����Ϊ��Cl2��HClO��ClO-��

 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�