��Ŀ����
����Ŀ��������Ԫ�����ڱ���һ���֡�
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
һ | A | |||||||
�� | D | J | L | |||||
�� | B | C | E | G |
��������Ԫ�������ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ��д�հס�
(1)�ǽ�������ǿ��Ԫ����_____________����ѧ��������õ���__________����L�⣬ԭ�Ӱ��������____________��A��D�γɵ�10����������_____________��/span>
(2)��������������������ǿ��˳��B��C��D��E����Ԫ�ص�����������Ӧˮ�������ѧʽ���У�___________��
(3)BԪ�������γɵļȺ������Ӽ����ֺ����ۼ��Ļ�����ĵ���ʽΪ____________��
(4)B��C��G��J����Ԫ�صļ����ӵİ뾶�ɴ�С��˳��Ϊ_________________��
���𰸡�F Ne Na ![]() NaOH Al(OH)3 H3PO4 HNO3
NaOH Al(OH)3 H3PO4 HNO3 ![]() Cl- ��F- ��Na+ ��Al3+
Cl- ��F- ��Na+ ��Al3+
��������
�����ڱ��Ľṹ֪AΪH��BΪNa��CΪAl��DΪN��EΪP��GΪCl��JΪF��LΪNe���ݴ˲����Ԫ����������գ�
(1)�����Ϸ�������ЩԪ���У��ǽ�������ǿ��Ԫ���Ƿ���ϡ������ԭ�����������������ѧ���ʲ����ã��ʻ�ѧ��������õ����ʣ���Ϊͬһ�����к˵��������ԭ�Ӱ뾶�ݼ�����ͬһ������Ӳ���Խ��ԭ�Ӱ뾶Խ�ʳ�L����ԭ���⣬ԭ�Ӱ뾶�������ƣ�A��D�γɵ�10����������笠����ӣ�
����F��Ne��Na��![]() ��
��
(2)������Խǿ�����������ˮ����ļ���Խǿ���ǽ�����Խǿ�����������ˮ���������Խǿ�������NaOH��Al(OH)3������H3PO4��HNO3�����Ϊ��NaOH��Al(OH)3��H3PO4��HNO3��
(3)BԪ�������γɵĻ������������ơ��������ƣ���Ⱥ������Ӽ����ֺ����ۼ��Ļ�����ĵ���ʽΪ![]() ��
��
����![]() ��
��
(4) BΪNa��CΪAl��GΪCl��JΪF�����������ӡ������Ӻͷ���������ͬ�ĵ��Ӳ�ṹ���˵����Խ�����Ӱ뾶ԽС��������ӵİ뾶F- ��Na+ ��Al3+����ͬһ������Ӳ���Խ�����Ӱ뾶Խ�������Ӱ뾶Cl- ��F-�����ɴ�С��˳��ΪCl- ��F- ��Na+ ��Al3+��
����Cl- ��F- ��Na+ ��Al3+��

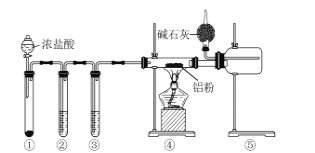
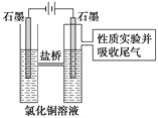
����Ŀ��ij����С������ͼ��ʾ��ʵ��װ��̽�������백��֮��ķ�Ӧ������A��FΪ�����������ķ���װ�ã�CΪ����������������백����Ӧ��װ�á�
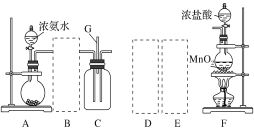
��ش��������⣺
(1)װ��A�е���ƿ�ڹ����ѡ��________(����ĸ)��
A.��ʯ�ҡ� B.Ũ���ᡡ C.��ʯ�ҡ� D.���������� E.�ռ�
(2)���߿���Ӧ���ӱ�Ҫ�ij���װ�ã������ͼ�ı�ѡװ����ѡ��������������пո�
��ѡװ�� | ||
|
|
|
�� | �� | �� |
B��________��D��________��E��________��
(3)�����Ͱ����ڳ����»�Ͼ��ܷ�����Ӧ�����Ȼ�狀͵������÷�Ӧ�Ļ�ѧ����ʽΪ��_______
(4)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ�����һ��ʵ�鷽��ȷ���ð�ɫ�����е������ӣ�________________