��Ŀ����
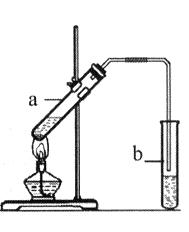
������Ӧ�Ǽ���ȩ������Ҫ��Ӧ���̲ĶԸ�ʵ��IJ�������ֻ�Ǵ��Ե�������ijͬѧ���������о���
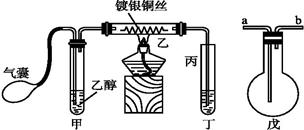
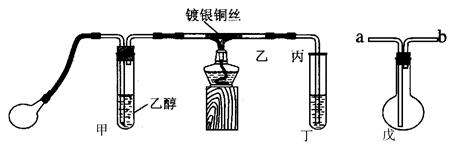
��1���ڸɾ��Թ��м���2ml2�� ��Ȼ�� �õ�������Һ����װ��5֧�Թܣ����Ϊ1#��2#��3#��4#��5# ��
��2�����εμ�2%��5%��10%��20%��40%����ȩ��Һ4�Σ�����������60��~70���ˮԡ�С�3���Ӻ��Թ�1#δ�γɴ�����������Թ�5# �������������кڰߣ� �Թ�4# ��������������һ�㣬�Թ�2#��3# �γɹ�����������
���о���Ŀ���ǣ� ��
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����������:��ǿ���������£�����������Һ����������������֤�ͶԱ�ʵ�����¡�
| װ�� | ʵ����� | �Թ��е�ҩƷ | ���� |
 | ʵ��� | 2mL������Һ�����ν�ŨNaOH��Һ | �����ݲ���: һ��ʱ�����Һ���:�Թܱڸ������� |
| ʵ��� | 2mL������Һ�� ����Ũ��ˮ | �����ݲ���:һ��ʱ�����Һ�����Ա仯 |
��ͬѧ������ʵ����ʵ���IJ��죬�������Ϻ�֪:
a��Ag(NH3)2++2H2O
 Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O�����飬ʵ������������NH3,��ɫ��������Ag2O������Ag2O��ԭ���ǣ�
�������������������� ��
��5����ʪ��ĺ�ɫʯ����ֽ����NH3�������������������������������������������� ����ϡHNO3�� ϴ�Թܱ��ϵ�Ag���÷�Ӧ�Ļ�ѧ����ʽ������������������ �� �������� ��
��6����ͬѧ�Բ���������ԭ���������:��NaOH�����£�������NH3��ԭAg2O������ü����������˵������ȩ��ʱ��������Һ���ܳ�ǿ���ԣ���Ϊ��
��1��AgNO3��Һ, ��μ���ϡ��ˮ,ֱ����������İ�ɫ������ʧΪֹ��
��2��̽��������Ӧʵ���У���ȩ��Һ��Ũ��������Ĺ�ϵ
��3��
��4����NaOH�����£�����ʹNH3�ݳ�����ʹƽ��Ag(NH3)2 + + 2H2O �� ��Ag++ 2NH3��H2O�����ƶ���c��Ag+������Ag+��OH����Ӧ����ת��ΪAg2O��
��Ag++ 2NH3��H2O�����ƶ���c��Ag+������Ag+��OH����Ӧ����ת��ΪAg2O��
��5����ֽ����; 4HNO3��ϡ��+ 3Ag��3AgNO3+ NO��+2H2O
��6����ǿ���������£�������Һ����Ҳ���γ����������뺬ȩ��������
���������������2���ı���ȩŨ�ȣ��۲��������ԣ�̽��������Ӧʵ���У���ȩ��Һ��Ũ��������Ĺ�ϵ��
��4��Fe2��������������Fe3����Fe3����KSCN��Ӧ����Ѫ��ɫ��Fe2�����ܡ�
���㣺�����Ի�ѧʵ��Ϊ����������Ԫ�ؼ��仯�����ʵ���֪ʶ��

 �żӾ���ϵ�д�
�żӾ���ϵ�д����ⶨ�Ҵ��Ļ�ѧʽ��C2H6O�������л����ձ���ڵ�ͬ���칹�����Ʋ��Ҵ��Ľṹ��������������֮һ��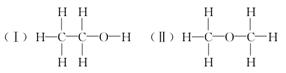
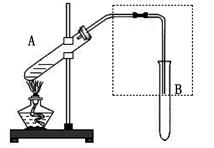
Ϊ�ⶨ��ṹ��Ӧ�������ʵ������Խ��ж��ԡ�����ʵ�顣�ָ����Ҵ����ơ�ˮ����Ҫ����������ס��ҡ�����������ͬѧֱ��������ͼ����װ�ý���ʵ��ȷ���Ҵ��Ľṹ��
(1)ѧ���õ�һ��ʵ�����ݣ�
| �Ҵ������ʵ���(mol) | ���������(L) |
| 0.10 | 1.12(��״��) |
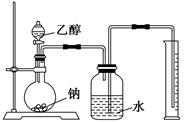
�������������ƶ��Ҵ��ĽṹӦΪ________(�â��ʾ)������Ϊ_______________��
(2)ͬѧ�ҷֱ�ȷ����4.60 g�Ҵ����ж��ʵ�飬����������ŵ���Ͳ�ڵ�ˮ�������Ϊ���ɵ�H2�������ɱ�״����С��1.12 L�����������Ͳ�������Ҷ�����ɵ�����ô����Ϊ������������Ʒ�к�������ˮ��ɵģ�����Ϊ��ȷ��________(���ȷ������ȷ��)�������Ϊ��ȷ����˵�����ɣ��������Ϊ����ȷ���Dz������������ԭ��Ӧ����ʲô��________________��
(3)ͬѧ����Ϊʵ��ɹ��Ĺؼ��У���װ��������Ҫ����
��ʵ�鿪ʼǰȷȷ���Ҵ������������������ܹ��ƿ��ˮ�������������������IJ��㷽����ȷ������ȷ��������ȷ����________��(�����)
(4)ͬѧ������ͨ�������Ҵ���������ȷ���Ҵ���������ô������֪����������_____________��
(5)ʵ�������ͬѧ���Ҵ��Ŀ��ܽṹ�������ֶ��Ҵ����Ƶ����Ĺ�ϵ���������ۣ�����Ҵ������ʵ���Ϊn mol����ô���Ƶ����ʵ�����ȡֵҪ�������_________________��
���з�Ӧ�У�����ȡ����Ӧ����
A��  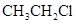 |
B��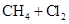   |
C��   |
D�� 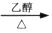 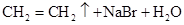 |
�ṹΪ����CH��CH��CH��CH��CH��CH��CH��CH�����ĸ߷��ӻ������õ������������䵼�����������ߡ������߷��ӻ�����ĵ�����
| A����Ȳ | B����ϩ | C����ϩ | D��1,3������ϩ |
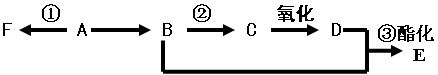


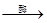 2________+2C2H5OH
2________+2C2H5OH