��Ŀ����
ij����С��������ͼװ�ý����Ҵ��Ĵ�����ʵ�鲢��ȡ��ȩ��ͼ������̨��װ�ü���ȥ���ֺ��߱�ʾ�齺�ܡ�����д���пհף�
��1����װ�ó��������¶�Ϊ70��80���ˮԡ�У�Ŀ���� ��
��2��ʵ��ʱ���ȼ��Ȳ��������еĶ�����˿��Լ1���Ӻ�����������ʱͭ��˿���ʺ���״̬�����Ѿƾ��Ƴ��ߣ�����һ���Ĺ����ٶȣ�ͭ˿�ܳ�ʱ�䱣�ֺ���ֱ��ʵ�������
��3���Ҵ��Ĵ�������Ӧ�� ��Ӧ������ȡ������ȡ������÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����ʵ������п��ƹ������ٶȺ���Ҫ:
�ٿ��ƹ����ٶȵķ����� ��
���������ٶȹ��췴Ӧ��ֹͣ,ԭ�� ��
���������ٶȹ�����ӦҲ��ֹͣ,ԭ�� ��
��5�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã������ӷ����ǣ�����װ���е��ܴ��ţ����ҽ� �� �ӱ���
��1���ʵ��ӿ������Ҵ����������ʣ����ƽ�ȵ��Ҵ���������3�����ȣ�2CH3CH2OH��O2 2CH3CHO��2H2O����4���ٿ��Ƽ��е�λʱ�������������ڴ��߹������������Ա�֤��Ӧ�����¶ȣ��۷�Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶ȣ�5��b��a
2CH3CHO��2H2O����4���ٿ��Ƽ��е�λʱ�������������ڴ��߹������������Ա�֤��Ӧ�����¶ȣ��۷�Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶ȣ�5��b��a
���������������1����װ�ó��������¶�Ϊ70��80���ˮԡ�У�ˮԡ��ʹ�������Ⱦ��ȣ���ʹ�����Ҵ�ƽ���������Ҵ��������ʴ�Ϊ���ʵ��ӿ������Ҵ����������ʣ����ƽ�ȵ��Ҵ���������3��ʵ��ʱ���ȼ��Ȳ��������еĶ�����˿��Լ1���Ӻ�����������ʱͭ˿���ʺ���״̬�����Ѿƾ��Ƴ��ߣ�����һ���Ĺ����ٶȣ�ͭ˿�ܳ�ʱ�䱣�ֺ���ֱ��ʵ�������˵����Ӧ����������ȼ��ɽ��е��ף�˵���÷�Ӧ�Ƿ��ȵķ�Ӧ���Ҵ�����������������ȩ��ˮ���ʴ�Ϊ�����ȣ�2CH3CH2OH+O2 2CH3CHO+H2O����3���ٹ������ٶ�Խ�죬���е�λʱ����ð���������Խ�࣬��֮��Խ�٣��ʴ�Ϊ�����Ƽ��е�λʱ�������������ڹ����ٶȹ�����������������߹������������Ա�֤��Ӧ�����¶ȣ����·�Ӧֹͣ���ʴ�Ϊ�������ٶȹ�����������������߹������������Ա�֤��Ӧ�����¶ȣ��۹����ٶȹ�����Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶ȣ����·�Ӧֹͣ���ʴ�Ϊ����Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶ȣ���4��Ϊ��ֹ��ֹ����ˮ��������ȫƿ�еĵ������ǡ��̽������������Գ������л������ã��ʴ�Ϊ��b��a��
2CH3CHO+H2O����3���ٹ������ٶ�Խ�죬���е�λʱ����ð���������Խ�࣬��֮��Խ�٣��ʴ�Ϊ�����Ƽ��е�λʱ�������������ڹ����ٶȹ�����������������߹������������Ա�֤��Ӧ�����¶ȣ����·�Ӧֹͣ���ʴ�Ϊ�������ٶȹ�����������������߹������������Ա�֤��Ӧ�����¶ȣ��۹����ٶȹ�����Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶ȣ����·�Ӧֹͣ���ʴ�Ϊ����Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶ȣ���4��Ϊ��ֹ��ֹ����ˮ��������ȫƿ�еĵ������ǡ��̽������������Գ������л������ã��ʴ�Ϊ��b��a��
���㣺�Ҵ��Ļ�ѧ���ʣ��������鷽�������

 ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�ij��ѧ����С��̽������ˮ���������������������ʵ�顣
��1��ʵ����Ʒ��0.5 g���ۡ�4 mL 20%������Һ������������Һ��������Һ����ˮ��
���裺���Թ�1���Թ�2�������0.5 g���ۣ����Թ�1�����4 mL 20%������Һ�����Թ�2�����4 mLˮ��������3��4 min���ù�����Һ�к��Թ�1���������Һ����һ����Һ�嵹���Թ�3�����Թ�2��3�ﶼ�����ˮ���۲���û����ɫ���֡����Թ�1�����������Һ���Լ��Ⱥ۲��Թ��ڱ��������������֡�
������ѧ֪ʶԤ����ܵ�ʵ������
ʵ����������ۣ������±�����
| �Թ� | �����ˮ | ����������Һ | ���� |
| 1 | | | |
| 2 | | | |
| 3 | | | |
��2��ʵ����Ʒ��0.5 g���ۡ���Һ������������ͭ����Һ��
���裺���Թ��м���0.5 g���ۣ�����������Һ���ȣ���ˮ��Һ�м�������������ͭ����Һ������У��۲�����
����__________________________________________��
���ۣ�__________________________________________��
��3���Ƚ�ʵ�飨1����2���ɵó��Ľ��ۣ�___________________��
������Ӧ�Ǽ���ȩ������Ҫ��Ӧ���̲ĶԸ�ʵ��IJ�������ֻ�Ǵ��Ե�������ijͬѧ���������о���
��1���ڸɾ��Թ��м���2ml2�� ��Ȼ�� �õ�������Һ����װ��5֧�Թܣ����Ϊ1#��2#��3#��4#��5# ��
��2�����εμ�2%��5%��10%��20%��40%����ȩ��Һ4�Σ�����������60��~70���ˮԡ�С�3���Ӻ��Թ�1#δ�γɴ�����������Թ�5# �������������кڰߣ� �Թ�4# ��������������һ�㣬�Թ�2#��3# �γɹ�����������
���о���Ŀ���ǣ� ��
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����������:��ǿ���������£�����������Һ����������������֤�ͶԱ�ʵ�����¡�
| װ�� | ʵ����� | �Թ��е�ҩƷ | ���� |
 | ʵ��� | 2mL������Һ�����ν�ŨNaOH��Һ | �����ݲ���: һ��ʱ�����Һ���:�Թܱڸ������� |
| ʵ��� | 2mL������Һ�� ����Ũ��ˮ | �����ݲ���:һ��ʱ�����Һ�����Ա仯 |
��ͬѧ������ʵ����ʵ���IJ��죬�������Ϻ�֪:
a��Ag(NH3)2++2H2O
 Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O�����飬ʵ������������NH3,��ɫ��������Ag2O������Ag2O��ԭ���ǣ�
�������������������� ��
��5����ʪ��ĺ�ɫʯ����ֽ����NH3�������������������������������������������� ����ϡHNO3�� ϴ�Թܱ��ϵ�Ag���÷�Ӧ�Ļ�ѧ����ʽ������������������ �� �������� ��
��6����ͬѧ�Բ���������ԭ���������:��NaOH�����£�������NH3��ԭAg2O������ü����������˵������ȩ��ʱ��������Һ���ܳ�ǿ���ԣ���Ϊ��
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
��װ���е���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ ����
�Ƹ÷�Ӧ�������෴Ӧ���� ��
| A���ӳɷ�Ӧ | B��ȡ����Ӧ | C��ˮ�ⷴӦ | D��������Ӧ |
��д����ȡ���������Ļ�ѧ��Ӧ����ʽ��
ij���������۾�����Ϊ����ͼ���ҵľۺ�������й�˵���������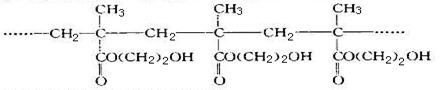
| A�����ɸþۺ���ķ�Ӧ���Ӿ۷�Ӧ |
| B���þۺ���ĵ����DZ������� |
| C��i�ۺ�������д��ڴ����ġ�OH�����Ծ��нϺõ���ˮ�� |
D���þۺ���ĽṹͲʽΪ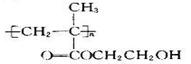 |
��ɫ��ѧ�Ի�ѧ��Ӧ����ˡ�ԭ�Ӿ����ԡ� ���¸��Ҫ�������ԭ�Ӿ����Է�Ӧ��ԭ�Ϸ����е�ԭ��ȫ��ת��������Ҫ�IJ���,������������,ʵ�����ŷš����м��������ұ��ķ�����,ԭ�Ӿ�������õ���(��Ӧ����һ�������½���) (����)
A�� +C2H5Cl +C2H5Cl  +HCl +HCl |
B�� +C2H5OH +C2H5OH  +H2O +H2O |
C�� +CH2 +CH2 CH2 CH2  |
D��   +HBr +HBr |
 +H2
+H2

���з�Ӧ���ͣ�����������ȡ��������ȥ���ܼӳɡ���ˮ�⡢��ԭ���������л�������������ǻ�����
| A���٢ڢۢ� | B���ڢܢ� | C���ڢܢݢ� | D���ڢܢ� |



