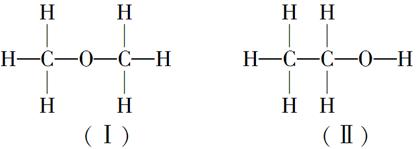��Ŀ����
���ⶨ�Ҵ��Ļ�ѧʽ��C2H6O�������л����ձ���ڵ�ͬ���칹�����Ʋ��Ҵ��Ľṹ��������������֮һ��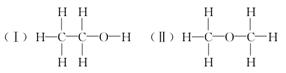
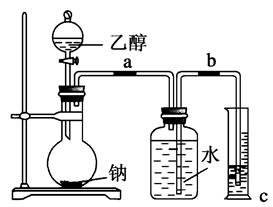
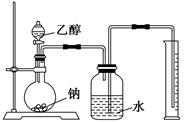
Ϊ�ⶨ��ṹ��Ӧ�������ʵ������Խ��ж��ԡ�����ʵ�顣�ָ����Ҵ����ơ�ˮ����Ҫ����������ס��ҡ�����������ͬѧֱ��������ͼ����װ�ý���ʵ��ȷ���Ҵ��Ľṹ��
(1)ѧ���õ�һ��ʵ�����ݣ�
| �Ҵ������ʵ���(mol) | ���������(L) |
| 0.10 | 1.12(��״��) |
�������������ƶ��Ҵ��ĽṹӦΪ________(�â��ʾ)������Ϊ_______________��
(2)ͬѧ�ҷֱ�ȷ����4.60 g�Ҵ����ж��ʵ�飬����������ŵ���Ͳ�ڵ�ˮ�������Ϊ���ɵ�H2�������ɱ�״����С��1.12 L�����������Ͳ�������Ҷ�����ɵ�����ô����Ϊ������������Ʒ�к�������ˮ��ɵģ�����Ϊ��ȷ��________(���ȷ������ȷ��)�������Ϊ��ȷ����˵�����ɣ��������Ϊ����ȷ���Dz������������ԭ��Ӧ����ʲô��________________��
(3)ͬѧ����Ϊʵ��ɹ��Ĺؼ��У���װ��������Ҫ����
��ʵ�鿪ʼǰȷȷ���Ҵ������������������ܹ��ƿ��ˮ�������������������IJ��㷽����ȷ������ȷ��������ȷ����________��(�����)
(4)ͬѧ������ͨ�������Ҵ���������ȷ���Ҵ���������ô������֪����������_____________��
(5)ʵ�������ͬѧ���Ҵ��Ŀ��ܽṹ�������ֶ��Ҵ����Ƶ����Ĺ�ϵ���������ۣ�����Ҵ������ʵ���Ϊn mol����ô���Ƶ����ʵ�����ȡֵҪ�������_________________��
(1)�ó��Ҵ���������һ��H���������H��ͬ���Ӷ�ȷ���Ҵ����ӵĽṹΪ��(2)����ȷ�����ƿ����Ͳ֮�䲣��������ˮ�������û��������
(3)�٢ڢۢݡ�(4)�����Ҵ���Ʒ���ܶȡ�(5)����n mol
����

 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��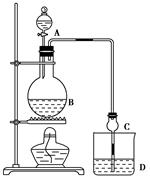
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/ �� | 34.7 | 78.5 | 118 | 77.1 |
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��1��Ũ����������� ������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ�� ��
���θ����C�������� ������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ����(�����ӷ���ʽ��ʾ�� ����Ӧ������D�е������� ��
��3����D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ������ ��
������Ӧ�Ǽ���ȩ������Ҫ��Ӧ���̲ĶԸ�ʵ��IJ�������ֻ�Ǵ��Ե�������ijͬѧ���������о���
��1���ڸɾ��Թ��м���2ml2�� ��Ȼ�� �õ�������Һ����װ��5֧�Թܣ����Ϊ1#��2#��3#��4#��5# ��
��2�����εμ�2%��5%��10%��20%��40%����ȩ��Һ4�Σ�����������60��~70���ˮԡ�С�3���Ӻ��Թ�1#δ�γɴ�����������Թ�5# �������������кڰߣ� �Թ�4# ��������������һ�㣬�Թ�2#��3# �γɹ�����������
���о���Ŀ���ǣ� ��
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����������:��ǿ���������£�����������Һ����������������֤�ͶԱ�ʵ�����¡�
| װ�� | ʵ����� | �Թ��е�ҩƷ | ���� |
 | ʵ��� | 2mL������Һ�����ν�ŨNaOH��Һ | �����ݲ���: һ��ʱ�����Һ���:�Թܱڸ������� |
| ʵ��� | 2mL������Һ�� ����Ũ��ˮ | �����ݲ���:һ��ʱ�����Һ�����Ա仯 |
��ͬѧ������ʵ����ʵ���IJ��죬�������Ϻ�֪:
a��Ag(NH3)2++2H2O
 Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O�����飬ʵ������������NH3,��ɫ��������Ag2O������Ag2O��ԭ���ǣ�
�������������������� ��
��5����ʪ��ĺ�ɫʯ����ֽ����NH3�������������������������������������������� ����ϡHNO3�� ϴ�Թܱ��ϵ�Ag���÷�Ӧ�Ļ�ѧ����ʽ������������������ �� �������� ��
��6����ͬѧ�Բ���������ԭ���������:��NaOH�����£�������NH3��ԭAg2O������ü����������˵������ȩ��ʱ��������Һ���ܳ�ǿ���ԣ���Ϊ��
���з�Ӧ������ȡ����Ӧ����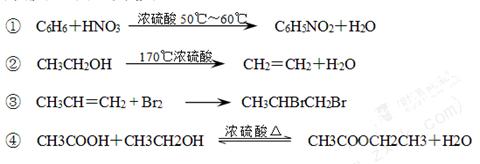
| A���٢� | B���ۢ� | C���٢� | D���٢� |
��8�����ʣ��ټ��飻�ڱ����۾���ϩ����1,3-����ϩ����2-��Ȳ�������飻���ڶ��ױ������ϩ������ʹ���Ը��������Һ��ɫ��������ˮ������ѧ��Ӧʹ֮��ɫ���� �� ��
| A���ۢܢݢ� | B���ܢݢߢ� | C���ܢݢ� | D���ۢܢݢߢ� |
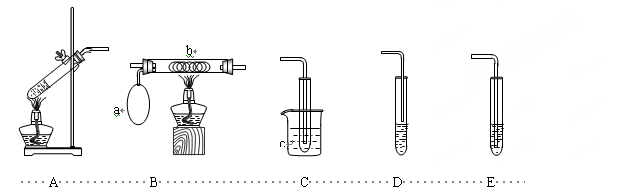
 �������Ҵ����Ƶķ�Ӧ�������ͼװ�ý���ʵ�飬����ƿ�з����������ƣ��ӷ�Һ©���л�������һ�������Ҵ���ͨ��������Ͳ��ˮ��������Ϳ�֪��Ӧ���ɵ������������
�������Ҵ����Ƶķ�Ӧ�������ͼװ�ý���ʵ�飬����ƿ�з����������ƣ��ӷ�Һ©���л�������һ�������Ҵ���ͨ��������Ͳ��ˮ��������Ϳ�֪��Ӧ���ɵ������������