��Ŀ����
ij��ѧС���Ա�����Ϊԭ����ȡ������������й����ʵķе����Է������������
I.�ϳɱ���������ֲ�Ʒ
����ƿ�м���12.2g�������20mL�״����ܶ�Լ0.79g/mL�� ����С�ļ���3mL Ũ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
��1���÷�Ӧ��Ũ��������� ������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ ���״�������ԭ�� ��
��2���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������ ��
��3���ס��ҡ�����λͬѧ�ֱ��������ͼ����ʵ������ȡ�����������װ�ã��г������ͼ�������������ȥ���������л�����ص㣬��ò��� װ�ã���ס������ҡ�������������
�ֲ�Ʒ�ľ���
��4������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ���������������ͼ���о��ƣ����������ͼ����ǡ���������������ƣ�����IΪ ������IIΪ ��
��5����������ͼ�м���Na2CO3��Һ�����Һ©���������ã�Ҫ�õ��л��㣬���������� ��
��6������������IJ���Ϊ ��
��1����������ˮ����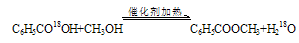 �÷�Ӧ�ǿ��淴Ӧ���״��ȱ�����������Ҽ״��е�ͣ�����ʧ�����Ӽ״�����������߲���
�÷�Ӧ�ǿ��淴Ӧ���״��ȱ�����������Ҽ״��е�ͣ�����ʧ�����Ӽ״�����������߲���
��2����ȴ��
��3����
��4����Һ������
��5������Һ©���ϿڵIJ���������ʹ���ϵİ��۶�©�����ϵ�С�ף�����������Һ©������������ƿ�����²�Һ�壬���²�Һ��պ�����ʱ�رջ��������л���ӷ�Һ©���Ͽڵ�����һ�ɾ������������ձ�����
��6��65 %
����

 ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/ �� | 34.7 | 78.5 | 118 | 77.1 |
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��1��Ũ����������� ������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ�� ��
���θ����C�������� ������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ����(�����ӷ���ʽ��ʾ�� ����Ӧ������D�е������� ��
��3����D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ������ ��
ij��ѧ����С��̽������ˮ���������������������ʵ�顣
��1��ʵ����Ʒ��0.5 g���ۡ�4 mL 20%������Һ������������Һ��������Һ����ˮ��
���裺���Թ�1���Թ�2�������0.5 g���ۣ����Թ�1�����4 mL 20%������Һ�����Թ�2�����4 mLˮ��������3��4 min���ù�����Һ�к��Թ�1���������Һ����һ����Һ�嵹���Թ�3�����Թ�2��3�ﶼ�����ˮ���۲���û����ɫ���֡����Թ�1�����������Һ���Լ��Ⱥ۲��Թ��ڱ��������������֡�
������ѧ֪ʶԤ����ܵ�ʵ������
ʵ����������ۣ������±�����
| �Թ� | �����ˮ | ����������Һ | ���� |
| 1 | | | |
| 2 | | | |
| 3 | | | |
��2��ʵ����Ʒ��0.5 g���ۡ���Һ������������ͭ����Һ��
���裺���Թ��м���0.5 g���ۣ�����������Һ���ȣ���ˮ��Һ�м�������������ͭ����Һ������У��۲�����
����__________________________________________��
���ۣ�__________________________________________��
��3���Ƚ�ʵ�飨1����2���ɵó��Ľ��ۣ�___________________��
������Ӧ�Ǽ���ȩ������Ҫ��Ӧ���̲ĶԸ�ʵ��IJ�������ֻ�Ǵ��Ե�������ijͬѧ���������о���
��1���ڸɾ��Թ��м���2ml2�� ��Ȼ�� �õ�������Һ����װ��5֧�Թܣ����Ϊ1#��2#��3#��4#��5# ��
��2�����εμ�2%��5%��10%��20%��40%����ȩ��Һ4�Σ�����������60��~70���ˮԡ�С�3���Ӻ��Թ�1#δ�γɴ�����������Թ�5# �������������кڰߣ� �Թ�4# ��������������һ�㣬�Թ�2#��3# �γɹ�����������
���о���Ŀ���ǣ� ��
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����������:��ǿ���������£�����������Һ����������������֤�ͶԱ�ʵ�����¡�
| װ�� | ʵ����� | �Թ��е�ҩƷ | ���� |
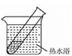 | ʵ��� | 2mL������Һ�����ν�ŨNaOH��Һ | �����ݲ���: һ��ʱ�����Һ���:�Թܱڸ������� |
| ʵ��� | 2mL������Һ�� ����Ũ��ˮ | �����ݲ���:һ��ʱ�����Һ�����Ա仯 |
��ͬѧ������ʵ����ʵ���IJ��죬�������Ϻ�֪:
a��Ag(NH3)2++2H2O
 Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O�����飬ʵ������������NH3,��ɫ��������Ag2O������Ag2O��ԭ���ǣ�
�������������������� ��
��5����ʪ��ĺ�ɫʯ����ֽ����NH3�������������������������������������������� ����ϡHNO3�� ϴ�Թܱ��ϵ�Ag���÷�Ӧ�Ļ�ѧ����ʽ������������������ �� �������� ��
��6����ͬѧ�Բ���������ԭ���������:��NaOH�����£�������NH3��ԭAg2O������ü����������˵������ȩ��ʱ��������Һ���ܳ�ǿ���ԣ���Ϊ��
ij���������۾�����Ϊ����ͼ���ҵľۺ�������й�˵���������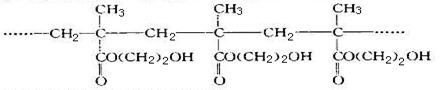
| A�����ɸþۺ���ķ�Ӧ���Ӿ۷�Ӧ |
| B���þۺ���ĵ����DZ������� |
| C��i�ۺ�������д��ڴ����ġ�OH�����Ծ��нϺõ���ˮ�� |
D���þۺ���ĽṹͲʽΪ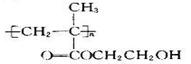 |
���з�Ӧ���ڼӳɷ�Ӧ����
| A��2CH3CH2OH + 2Na ��2CH3CH2ONa + H2�� |
B�� |
C�� |
D�� |





