��Ŀ����
15����������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã�Na2SO3+S$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3����������Һ�������ľ���ΪNa2S2O3•5H2O��Na2S2O3•5H2O��40��-45���ۻ���48��ֽ⣻Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ��������ͼ1��ʾ��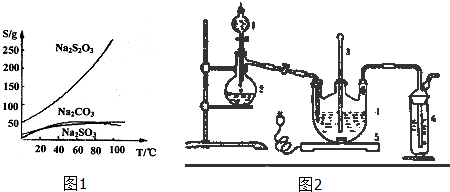
���ְ����·����Ʊ�Na2S2O3•5H2O��
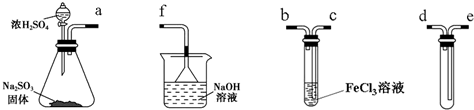
�����ƺ�̼���ư���ӦҪ�����һ������������ƿ�У�ע��150mL����ˮʹ���ܽ⣬�ڷ�Һ©���У�ע��Ũ���ᣬ��װ��2�м����������ƹ��壬������ͼ2��װ��װ�ã�
��1������2������Ϊ������ƿ��װ��6�пɷ���CD��
A��BaCl2��Һ B��ŨH2SO4 C������KMnO4��Һ D��NaOH��Һ
��2����Һ©��������ע��Ũ����ʹ��Ӧ�����Ķ�����������Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У����ô������������������ȣ���Ӧԭ��Ϊ��
��Na2CO3+SO2�TNa2SO3+CO2 ��Na2S+SO2+H2O�TNa2SO3+H2S
��2H2S+SO2�T3S��+2H2O ��Na2SO3+S$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3
�ܷ�ӦʽΪ��2Na2S+Na2CO3+4SO2�T3Na2S2O3+CO2
���Ŷ������������ͨ�룬������Һ���д���dz��ɫ��������������ͨ�����������壬��ӦԼ��Сʱ������Һ��pH�ӽ���С��7ʱ������ֹͣͨ���ͼ��ȣ���ҺpHҪ���Ʋ�С��7������S2O32-+2H+=S��+SO2��+H2O�������ӷ���ʽ��ʾ����
����Na2S2O3•5H2O���궨��Һ��Ũ�ȣ�

��1��Ϊ���ٲ�Ʒ����ʧ��������Ϊ���ȹ��ˣ��������dz���ϴ�Ӹ������ϴ�Ӳ��������Ҵ������Լ�����ϴ�Ӽ���
��2������Ũ����Һֱ����Һ����ɫ����Ϊֹ������ʱΪʲôҪ�����¶Ȳ��˹����¶ȹ��ᵼ�������ľ���ֽ⣮
��3����ȡһ�������IJ�Ʒ���ó������������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ��÷�����ƽȷ��ȡ������K2Cr2O7��Ħ������294g/moL��0.5880�ˣ�ƽ���ֳ�3�ݷֱ����3����ƿ�У���ˮ�����Һ�������������KI���ữ���������з�ӦCr2O72-+6I-+14H+�T2Cr3++7H2O+3I2���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��2S2O32-+I2�TS4O62-+2I-���ζ��յ������Ϊ��Һ����ɫǡ�ñ����ɫ���Ұ���Ӳ��ָ�����������Na2S2O3��Һ��ƽ�����Ϊ20.00mL�������궨�������������Һ��Ũ��Ϊ0.2000mol•L-1��
���� ��1�����������Ĺ�����ȷ������2�����ƣ�ʵ������SO2���ɣ���SO2�Ǵ�����Ⱦ����������ŷţ���Ҫβ�����������Ը��������Һ������������Һ���ԺͶ�������֮�䷴Ӧ��
��2�������������£����������������SO2��S��ˮ��
��1����������Ƶ��ܽ�����¶ȵ����߶����ͣ��ݴ�ȷ�����ʵķ��뷽����Na2S2O3������ˮ���������Ҵ�������ϴ��ʱҪע����������Ƶ���ģ�
��2������Na2S2O3•5H2O��40��45���ۻ���48��ֽ���������ش�
��3��������������ɫ�����ݷ����ķ�Ӧ���õ���ϵʽ��K2Cr2O7��3I2��6 Na2S2O3���ݴ˼��㼴�ɣ�
��� �⣺��1�����������Ĺ����֪������2������Ϊ������ƿ������ʵ������SO2���ɣ���SO2�Ǵ�����Ⱦ����������ŷţ���Ҫβ������������װ��6������������SO2����ֹ��Ⱦ������SO2������������Ҿ��л�ԭ�ԣ����Կ������Ը��������Һ������������Һ���գ�ѡCD��
�ʴ�Ϊ��������ƿ��CD��
��2����Ϊ�����������£����������������SO2��S��ˮ����Ӧ�����ӷ���ʽ��S2O32-+2H+=S��+SO2��+H2O��������ҺpHҪ���Ʋ�С��7��
�ʴ�Ϊ��S2O32-+2H+=S��+SO2��+H2O��
��1��������������Ƶ��ܽ�����¶ȵ����߶����ͣ����Բ���IӦ���dz��ȹ��ˣ�Na2S2O3������ˮ���������Ҵ�������Ϊ��ֹϴ����ʧ��������ƣ�Ӧ�����Ҵ���ϴ�Ӽ���
�ʴ�Ϊ�����ȹ��ˣ��Ҵ���
��2������Na2S2O3•5H2O��40��45���ۻ���48��ֽ⣬��������ʱҪ�����¶Ȳ��˹��ߵ�ԭ�����¶ȹ��ᵼ�������ľ���ֽ⣮
�ʴ�Ϊ���¶ȹ��ᵼ�������ľ���ֽ⣻
��3�����ڵ�����������ɫ�����Եζ��յ������Ϊ��Һ����ɫǡ�ñ����ɫ���Ұ���Ӳ��ָ������ݷ�Ӧ�ķ���ʽ��֪��
K2Cr2O7��3I2��6 Na2S2O3
1mol 6mol
$\frac{1}{3}$��$\frac{0.5880}{294}$mol c��0.02000L
���c=0.2000mol/L��
�ʴ�Ϊ����Һ����ɫǡ�ñ����ɫ���Ұ���Ӳ��ָ���0.2000��
���� ������ʵ��̽���ķ�ʽ����ѧ�����ʵķ�����ᴿ�����֪ʶ�������ۺ�֪ʶ�Ŀ��飬ע��֪ʶ�Ĺ��ɺ������ǽ���Ĺؼ����ѶȽϴ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��״���£�11.2L�������������ķ�����Ϊ0.5NA | |
| B�� | 1 mol���еĵ�����ĿΪ9NA | |
| C�� | 28g��ϩ�������õ��Ӷ���ĿΪ4NA | |
| D�� | ������ϩ����ϩ����ϩ�Ļ�����干14g����ԭ����ΪNA |
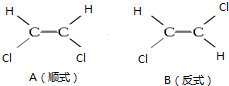 1��2һ������ϩ����ͼ���ֽṹ��
1��2һ������ϩ����ͼ���ֽṹ��
 ij�о�С��̽����
ij�о�С��̽���� ��ˮ���Ȼ�����SnCl4����һ����;�㷺���������м��壬��ƷΪ��ɫҺ�壬�۵�-33�棬�е�114.1�棬�ӷ�����ʪ��������ˮ������̣�ijͬѧ����������뾫����Ӧ�Ʊ���ˮ���Ȼ�����ʵ��װ����ͼ�����ּг�װ��δ��������
��ˮ���Ȼ�����SnCl4����һ����;�㷺���������м��壬��ƷΪ��ɫҺ�壬�۵�-33�棬�е�114.1�棬�ӷ�����ʪ��������ˮ������̣�ijͬѧ����������뾫����Ӧ�Ʊ���ˮ���Ȼ�����ʵ��װ����ͼ�����ּг�װ��δ��������