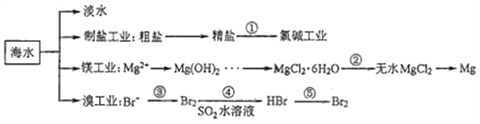
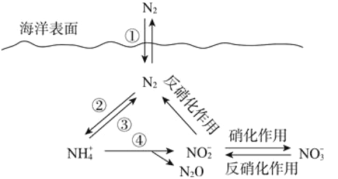
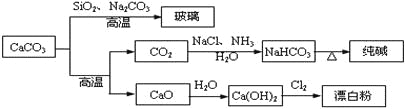
��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����ijһԪ�ء����ݱ�������Ԫ�ػش��������⣺

��1��Ԫ��d�����ڱ��е�λ����_____________��h��fԭ���������______________
��2��b��c��f�ļ����Ӱ뾶��С����___________�����������ţ���ԭ�Ӱ뾶��С����______���ѧʽ��
��3�����е�������Ԫ�صķǽ�������ǿ��_________����Ԫ�ط�����ʾ���� e��f��g����Ԫ�ص���̬�⻯����ȶ�����__________���û�ѧʽ��ʾ��
��4��gԪ����bԪ�ص�����������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽΪ________________________
����Ԫ�����ڱ������ǿ�����ʶ����Ԫ�ص�����
��5��Ԫ�����ڱ�λ�ڶԽ��ߵ�����Ԫ�����������Ƴ�֮Ϊ�Խ��߹��������ڱ��Խ��߹��ɣ�����Be���ʼ��仯����������������ʼ��仯������������ơ������Be(OH)2��Mg(OH)2��ѡ�õ��Լ�Ϊ__________��Һ
��6��Ǧ(Pb)������Sn�����ࣨGe����Ԫ�أ�C)���裨Si��ͬ���壬�������䵥���ڿ����У��������Ӧ��Ǧ��������һ������Ǧ�����������Ӧ�����������ᷴӦ���ɴ˿ɵó����½��ۣ�
�� ���ԭ������Ϊ__________��
�� Ǧ��Pb��������Sn�����ࣨGe����+4����������ļ�����ǿ������˳��Ϊ__________���ѧʽ��
��7��������¹���ѧ��ʵ�����ԭ�����峬����̬���Ե̬�Ŀ���ת�����óɹ��������Ӽ�����о�����������ش�ͻ�ơ���֪﨣�Rb����37��Ԫ�أ���������85������ͬ���塣�ش��������⣺
��������ڱ��е�λ��Ϊ___________________
��ͬ����Ԫ�ص�ͬ�������������ƣ���д����
AlCl3�������RbOH��Ӧ�����ӷ���ʽ��_____________________________
������墨���һ�ּ�����γɵĻ�Ͻ���50g������������ˮ��Ӧʱ���ų���״���µ�����22��4L�����ּ����������__________
A��Li(7) B��Na(23) C��K(40) D��Cs��133��
���𰸡��������ڢ�A�� 18 Mg2+ S Cl PH3 NaOH+HClO4=NaClO4+H2O NaOH 32 Pb(OH)4> Sn(OH)4> Ge(OH)4 �������ڢ�A�� Al3++4OH-= AlO2-+2H2O AB
��������
��Ԫ�������ڱ��е�λ�ÿ�֪��aΪN��bΪNa��CΪMg��dΪAl��eΪP��fΪS��gΪCl��hΪSe��
��1����������=���Ӳ�����������������������������
��2��f��������3�����Ӳ㣬b��c���������������Ӳ��Ҿ�����ͬ�ĵ��Ӳ�ṹ��ԭ�����������Ӱ뾶С��
��3��ͬһ���ڣ��������ң��ǽ���������ǿ��
��4��gԪ����bԪ�ص�����������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽΪ��NaOH+HClO4=NaClO4+H2O.
��5��Be(OH)2����������������ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ��
��6���� ���ԭ������Ϊ32��
��ͬһ����������£�����������ǿ������������ˮ����ļ�������ǿ ��
��7����﨣�Rb����37��Ԫ�أ�������ڱ��е�λ��Ϊ�������ڢ�A�塣
��﨣�Rb����37��Ԫ�أ���������85������ͬ���壬AlCl3�������RbOH��Ӧ�����ӷ���ʽΪ��Al3++4OH-= AlO2-+2H2O��
��墨�ˮ��Ӧ�Ļ�ѧ����ʽΪ2Rb+2H2O=2RbOH+H2�������������ʵ���Ϊ1mol����2M+2H2O=2MOH+ H2����
2 1
2 mol 1mol
�������ƽ��Ħ������Ϊ50g/2 mol=25g/mol��Rb��Ħ������Ϊ85g/mol������һ�������Ħ������һ��С��25g/mol��������һ�����������Li(7) ��Na(23)��
��Ԫ�������ڱ��е�λ�ÿ�֪��aΪN��bΪNa��CΪMg��dΪAl��eΪP��fΪS��gΪCl��hΪSe��
��1����������=���Ӳ�����������������������������Ԫ��d�����ڱ��е�λ���ǵ������ڢ�A�壬h��fԭ���������18 ���ʴ�Ϊ���������ڢ�A�壻18��
��2��f��������3�����Ӳ㣬b��c���������������Ӳ��Ҿ�����ͬ�ĵ��Ӳ�ṹ��ԭ�����������Ӱ뾶С���ʼ����Ӱ뾶��С����Mg2+��ԭ�Ӱ뾶��С����S���ʴ�Ϊ��Mg2+��S��
��3��ͬһ���ڣ��������ң��ǽ���������ǿ���ʵ�������Ԫ�صķǽ�������ǿ��Cl��eΪP��fΪS��gΪCl����̬�⻯����ȶ�����PH3���ʴ�Ϊ��Cl��PH3��
��4��gԪ����bԪ�ص�����������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽΪ��NaOH+HClO4=NaClO4+H2O���ʴ�Ϊ��NaOH+HClO4=NaClO4+H2O��
��5���������⣬����Be���ʼ��仯����������������ʼ��仯������������ƣ�����Be(OH)2����������������ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ������Be(OH)2��Mg(OH)2��ѡ�õ��Լ�ΪNaOH���ʴ�Ϊ��NaOH��
��6���� ���ԭ������Ϊ32���ʴ�Ϊ��32��
��ͬһ����������£�����������ǿ������������ˮ����ļ�������ǿ ����Ǧ��Pb��������Sn�����ࣨGe����+4����������ļ�����ǿ������˳��ΪPb(OH)4> Sn(OH)4> Ge(OH)4���ʴ�Ϊ��Pb(OH)4> Sn(OH)4> Ge(OH)4��
��7����﨣�Rb����37��Ԫ�أ�������ڱ��е�λ��Ϊ�������ڢ�A�壬�ʴ�Ϊ���������ڢ�A�塣
��﨣�Rb����37��Ԫ�أ���������85������ͬ���壬AlCl3�������RbOH��Ӧ�����ӷ���ʽΪ��Al3++4OH-= AlO2-+2H2O���ʴ�Ϊ��Al3++4OH-= AlO2-+2H2O��
��墨�ˮ��Ӧ�Ļ�ѧ����ʽΪ2Rb+2H2O=2RbOH+H2�������������ʵ���Ϊ1mol����2M+2H2O=2MOH+ H2����
2 1
2 mol 1mol
�������ƽ��Ħ������Ϊ50g/2 mol=25g/mol��Rb��Ħ������Ϊ85g/mol������һ�������Ħ������һ��С��25g/mol��������һ�����������Li(7) ��Na(23)���ʴ�Ϊ��AB��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�