��Ŀ����
����Ŀ��ʵ��������NaOH��������1.0 mol��L��1��NaOH��Һ240 mL��
(1)������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ������ڼ��㡡���ܽ⡡��ҡ�ȡ���ת�ơ���ϴ�ӡ��߶��ݡ�����ȴ����ҡ��
����ȷ�IJ���˳��Ϊ________________�������õ��IJ����������ձ�����ͷ�ιܡ�_________��
(2)ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ��ʾ���ձ���ʵ������Ϊ________g��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�________g NaOH��

(3)��ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ��������������д����

��________________________��
��_____________________________��
(4)�����ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���________(����ĸ)��
A������NaOH�Ѿ�����
B��������ƿ�м�ˮδ���̶���
C��������NaOH��Һ�������ձ���
D���ô������������ƽ��5.4 g NaOH(1 g����������)ʱ�����ˡ������������
���𰸡��ڢ٢ۢ�ݢޢ�ߢ� ��������250mL����ƿ 27.4 10.0 ��Һʱû���ò��������� ѡ����500 mL����ƿ B
��������
��1������1.0molL-1��NaOH��Һ240mL��ѡ250mL����ƿ��������Ʋ���������
��2������ʱ�������룬���m��cVM���㣻
��3�����ݲ��������á���Һ����ȷ����������ƿ�����
��4�����c��n/V���������������
��1�����Ʋ���Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ת��ϴ��Һ�����ݡ�ҡ�ȡ�װƿ��������ȷ�IJ���˳��Ϊ�ڢ٢ۢ�ݢޢ�ߢܣ���ʵ������õ��IJ����������ձ�����ͷ�ιܡ���������250mL����ƿ��
��2������ʱ�������룬��ͼ��֪λ�÷ŷ������ձ�������Ϊ20g+10g-2.6g��27.4g��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ��������ƹ��������m��NaOH����0.25L��1.0molL-1��40g/mol��10.0g��
��3��������ʾ��ͼ��֪�����Ϊ����Һʱû���ò�����������������240mL��Һ��Ӧѡ��250mL����ƿ��������500mL����ƿ��
��4��A������NaOH�Ѿ����⣬����ʵ�ʳ��������������������٣�Ũ��ƫ�ͣ�
B��������ƿ�м�ˮδ���̶��ߣ���Һ������٣�Ũ��ƫ�ߣ�
C��������NaOH��Һ�������ձ�����ʵ����ʵ������٣�Ũ��ƫ�ͣ�
D���ô������������ƽ��5.4 g NaOH(1 g����������)ʱ�����ˡ������������������ʵ�ʳ��������������������٣�Ũ��ƫ�ͣ�
��ѡB��

 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�����Ŀ��ij�ϳ�������Ҫ�ɷ���һ����̼�������������ںϳɼ���(CH3OCH3)�����ȼ�ϡ�����Ȼ����øúϳ��������п��ܷ����ķ�Ӧ�У�
��CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1��+206.1 kJ��mol-1
CO(g)+3H2(g) ��H1��+206.1 kJ��mol-1
��CH4(g)+CO2(g)![]() 2CO(g)+2H2(g) ��H2��+247.3 kJ��mol-1
2CO(g)+2H2(g) ��H2��+247.3 kJ��mol-1
��CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H3
CO2(g)+H2(g) ��H3
��ش��������⣺
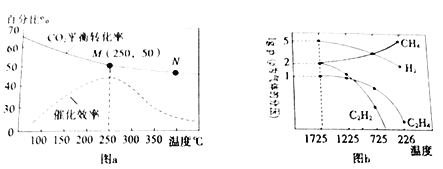
��1����һ�ܱ������н��з�Ӧ�������CH4�����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ��ʾ������10 minʱ���ı���������������__________________________��

��2����Ӧ������H3��_____________��800 ��ʱ����Ӧ����ƽ�ⳣ��K��1����ø��¶����ܱ�������ijʱ�̸����ʵ����ʵ������±���
CO | H2O | CO2 | H2 |
0.5 mol | 8.5 mol | 2.0 mol | 2.0 mol |
��ʱ��Ӧ���������淴Ӧ���ʵĹ�ϵʽ��_________(�����)��
a��v��>v�� b��v��<�� c��v����v�� d�����ж�
��3����ͼ2��ʾ���ڼס����������зֱ��������ʵ�����CH4��CO2��ʹ�ס�����������ʼ�ݻ���ȡ�����ͬ�¶��·�����Ӧ������ά�ַ�Ӧ�������¶Ȳ��䡣��֪��������CH4��ת������ʱ��ı仯��ͼ3��ʾ������ͼ3�л�����������CH4��ת������ʱ��仯��ͼ��___________

��4��ij�ϳ�������Ҫ�ɷ��е�һ����̼����һ��������Ҳ����NaOH��Һ��CO��Ӧ���ɼ�����(HCOONa)����һ����Ӧ���ɼ���������CO��Ⱦ�������½�a mol��COͨ��2 L b mol��L-1NaOH��Һ�У�ǡ����ȫ��Ӧ���ɼ����ƺͺ���������Ļ����Һ(������Һ�������)�������Һ��c(Na+)=c(HCOO-)����û����Һ�м���ĵ���ƽ�ⳣ��Ka=_________(�ú�a��b�Ĵ���ʽ��ʾ)��
����Ŀ����ҵ�Ͽ��ý�̿����������Ļ�����ڸ�������������Ӧ����SiCl4��SiCl4���ᴿ����������ԭ�øߴ��衣������ij��ѧС���Ʊ��ɾ�����������ʵ�����Ʊ�SiCl4�IJ���װ��ʾ��ͼ(ע: SiCl4��ˮ��ˮ��)��


��1��ѡ����ͼ����װ�ã��Ʊ������������������ȷ����������˳��Ϊ������װ�ü���___��____��_____��_____��װ��A(��Сд��ĸ���)��_____________
��2����ͼCװ�õ�������_______________��
��3��װ��A��Ӳ�ʲ������ж�������ͽ�̿������ǡ����ȫ��Ӧ����SCl4������������̼�����ʵ���֮��Ϊ_________________��
��4���������ۣ���С����ΪD������β��һ��ʱ����˹�����OH-������Һ�������ӿ϶�����Cl-��SO42-��ԭ����__________(�����ӷ���ʽ����)��
��5����ͬѧ��Ϊ���ܻ����������������(���Կ�����CO2��Ӱ��)��������м���(�����Ǹ����صĵ���)������1��ֻ��SO32-������2:ֻ��ClO-������3:����
���ʵ����֤��������1�ͼ���2��ȡ��������Һ���Թ��У��μ�3mol/LH2SO4����Һ�����ԣ�Ȼ��������Һ������a��b���Թ��У�����д�հ�ʵ�鲽�衢Ԥ������
ʵ�鲽��(������������) | Ԥ�ڬF�� | ���� |
��a�Թ��еμӼ���Ʒ����Һ���۲��������Թ�a���۲쵽 | ____________�� ____________�� | ����1���� |
��b�Թ��еμ�______________ | ��Һ��Ϊ��ɫ�� | ����2���� |