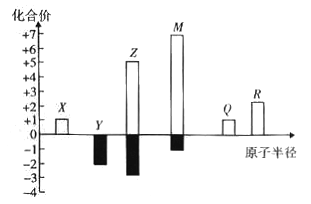
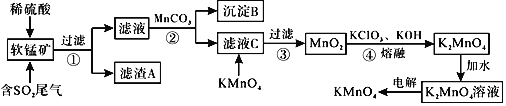
��Ŀ����
����Ŀ���Ӻ�ˮ�п���ȡ���ֻ���ԭ�ϣ������ǹ�ҵ�϶Ժ�ˮ�ɷ��ۺ����õ�ʾ��ͼ��
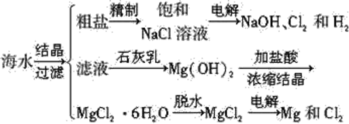
�Իش��������⣺
(1)д���ú�̲�ϵı�����Ca(OH)2�Ļ�ѧ����ʽ_________________________��
(2)д����ⱥ��NaCl��Һ�Ļ�ѧ����ʽ___________________________________��
(3)��������ɵ�����ͨ��ʯ�����п��Ƶ�Ư�ۣ�д���䷴Ӧ�Ļ�ѧ����ʽ_______________________��
(4)�ú�̲�ϵı�����Ca(OH)2���������ɽ�п���ʯ��ʯ��ȡ����Ҫ���ǵ�ʲô���⣿_________________________________��
���𰸡�CaCO3![]() CaO+CO2����CaO+H2O=Ca(OH)2 2NaCl+2H2O
CaO+CO2����CaO+H2O=Ca(OH)2 2NaCl+2H2O![]() 2NaOH+H2��+Cl2�� 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O �͵�ȡ�ģ����ͳɱ���
2NaOH+H2��+Cl2�� 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O �͵�ȡ�ģ����ͳɱ���
��������
(1)���ǵ���Ҫ�ɷ���̼��ƣ��ú�̲�ϵı�����ȡCa(OH)2�Ļ�ѧ����ʽΪCaCO3![]() CaO+CO2����CaO+H2O=Ca(OH)2��
CaO+CO2����CaO+H2O=Ca(OH)2��
(2)��ⱥ���Ȼ�����Һ������������ʧ������������������ˮ�������ӵõ��������������÷�Ӧ�Ļ�ѧ����ʽΪ��2NaCl+2H2O![]() 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����
(3)��������ɵ�����ͨ��ʯ�����пɷ�����Ӧ�������Ȼ��ƺʹ�����ƣ��Ƶ�Ư�ۣ��䷴Ӧ�Ļ�ѧ����ʽΪ2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O��
(4)�ú�̲�ϵı�����Ca(OH)2���ǿ��ǵ��͵�ȡ�ģ����ͳɱ����������ɽ�п���ʯ��ʯ��ȡ����Ҫ������������⣬���Ӳ�Ʒ�ijɱ���

����Ŀ����������������Ҫ�ľ�ϸ�����Լ�������������ˮ����ʳ���㾫��ʵ�����Ʊ��������£�

�Լ�����������±���
������ | �Ҵ� | ���������� | |
������״ | ��ɫ��״���� | ��ɫҺ�� | ��ɫ��Һ�� |
�е�/�� | 249.0 | 78.0 | 212.6 |
��Է����� | 122 | 46 | 150 |
�ܽ��� | ����ˮ���������Ҵ������ѵ��л��ܼ� | ��ˮ����Ȼ��� | ��������ˮ��������ˮ���������Ҵ������� |
�ش��������⣺
��1��Ϊ���ԭ�ϱ�����Ĵ��ȣ��ɲ��õĴ�������Ϊ_________��
��2������ٵ�װ����ͼ��ʾ�����Ⱥͼг�װ������ȥ������һС������������B�п��������״�������ˮ������ˮ����ͭ���Ҵ�������Һ����������B�У�������C�м��� 12.2 g������ı����ᾧ�壬30 mL��ˮ�Ҵ���Լ0.5 mol����3 mLŨ���ᣬ�����ʯ���������У�������Ӧ1.5~2 h������A��������_________������C�з�ӦҺӦ����_________��ʽ���ȡ�
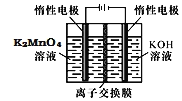
��3�����ŷ�Ӧ���У���Ӧ��ϵ��ˮ�ֲ��ϱ���Ч���룬����B����ˮ��������Ϊ_________��
��4����Ӧ������C�л��Һ���з����ᴿ������I��_________������II���õIJ������������ձ����_________��
��5����Ӧ����������н���ӦҺ������ˮ��Ŀ�ij����ܽ��Ҵ��⣬����_____�������Լ�XΪ_____����д��ѧʽ����
��6�����յõ����﴿Ʒ12.0 g��ʵ�����Ϊ_________ %��������λ��Ч���֣���
����Ŀ��������ͭ��Cu2O����һ����;�㷺�Ĺ����ϣ�ij��������ͭ��ʯ����CuFeS2��Cu2S�ȣ�Ϊԭ����ȡCu2O�Ĺ����������£�

�����¼������ʿ�ʼ�γɳ�������ȫ����ʱ��pH���±���
Fe��OH��2 | Fe��OH��3 | Cu��OH��2 | |
��ʼ���� | 7.5 | 2.7 | 4.8 |
��ȫ���� | 9.0 | 3.7 | 6.4 |
(1)¯���е��к�����ɷ���__________��Cu2S��O2��Ӧʱ���������뻹ԭ�������ʵ���֮��Ϊ__________��
(2)���Լ�X��H2O2��Һ��д����Ӧ��Ӧ�����ӷ���ʽ��________�����Լ�X��______ʱ���������ڽ��������ɱ���
(3)�����Լ�Y��pHʱ��pH�ĵ��ط�Χ��___________��
(4)д����N2H4�Ʊ�Cu2O�Ļ�ѧ����ʽ��________������X����_________��ϴ�ӡ���ɣ����к��ʱҪ������������Ŀ����____________��
(5)��ͭ��ʯī���缫�����Ũ��ǿ������Һ���Ƶ�����Cu2O��д������������Cu2O�ĵ缫��Ӧʽ��__________��
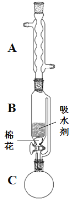
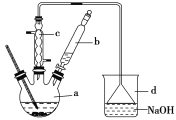
����Ŀ���屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
���� | �� | �� | �屽 |
�ܶ�/(g��cm��3) | 0.88 | 3.10 | 1.50 |
�е�/�� | 80 | 59 | 156 |
ˮ���ܽ�� | �� | �� | �� |
�����кϳɲ���ش����⣺
(1)��a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�塣��a�е��뼸���壬�а�ɫ��������������Ϊ������________���塣�����μ���Һ����ꡣװ��d��������___________��
(2)Һ�����������в�������ᴿ��
����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10mLˮ��8mL10%��NaOH��Һ��10mLˮϴ�ӡ�NaOH��Һϴ�ӵ�������___________________;
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˡ������Ȼ��Ƶ�Ŀ����____________;
(3)�����Ϸ���������屽�л����е���Ҫ����Ϊ________��Ҫ��һ���ᴿ�����в����б����õ�����________(����ĸ���)��
a���ؽᾧb������c������d����ȡ