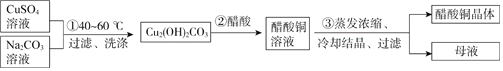
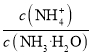ЬтФПФкШн
ЁОЬтФПЁПТЬЩЋжВЮяБъБОгУДзЫсЭЃл(CH3COO)2CuЃнДІРэКѓбеЩЋИќЯЪбоЁЂЮШЖЈЁЃФГЛЏбЇаЁзщжЦБИДзЫсЭОЇЬхВЂВтЖЈВњЦЗжаЭЕФКЌСПЃЌЪЕбщШчЯТЁЃ
Ђё.ДзЫсЭОЇЬхЕФжЦБИжа
(1)гУРызгЗНГЬЪНБэЪОЙ§ГЬЂйВњЮяжаOH-ЕФРДдДЃК___________ЃЛ
(2)Й§ГЬЂкЖдгІЕФЛЏбЇЗНГЬЪНЪЧЃК___________ЁЃ
Ђђ.ВтЖЈВњЦЗжаЭЕФКЌСП
i.ШЁagДзЫсЭВњЦЗЗХШыДјФЅПкШћзгЕФзЖаЮЦПжаЃЌгУЯЁДзЫсШмНтЃЌМгШыЙ§СПKIШмвКЃЌВњCulГСЕэЃЌШмвКГЪзиЛЦЩЋЃЛ
ii.гУb molЁЄL -1Na2S2O3БъзМШмвКЕЮЖЈiжаЕФзЧвКжСЧГЛЦЩЋЪБЃЌМгШыМИЕЮЕэЗлШмвКЃЌШмвКБфРЖЃЌМЬајгУNa2S2O3БъзМШмвКЕЮЖЈжСРЖЩЋНќгкЯћЪЇЃЛ(вбжЊЃК2![]() +I2ЃН
+I2ЃН![]() +2I-)
+2I-)
iii.ЯђЂЂЫљЕУзЧвКжаМгШыKSCNШмвКЃЌГфЗжвЁЖЏЃЌШмвКРЖЩЋМгЩюЃЛ
iv.МЬајгУNa2S2O3БъзМШмвКЕЮЖЈжазЧвКжСжеЕуЃЌЯћКФБъзМШмвКvmLЁЃ
вбжЊЃКЂйNa2S2O3ШмвККЭNa2S4O6ШмвКбеЩЋОљЮЊЮоЩЋЃЛ
ЂкCulвзЮќИНI2ЃЌБЛЮќИНЕФI2ВЛгыЕэЗлЗЂ CuSCNЩњЯдЩЋЗДгІЁЃФбШмгкЫЎЧвВЛЮќИНI2
(3)iжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЪЧ_________ЃЛ
(4)ДзЫсЭВњЦЗжаЭдЊЫиЕФжЪСПЗжЪ§ЪЧ_______ЁЃ
ЁОД№АИЁП![]() +H2OOH-+
+H2OOH-+![]() Cu2(OH)2CO3ЃЋ4CH3COOH=2(CH3COO)2CuЃЋ3H2OЃЋCO2Ёќ 2Cu2+ЃЋ4I- = 2CuIЁ§ЃЋI2
Cu2(OH)2CO3ЃЋ4CH3COOH=2(CH3COO)2CuЃЋ3H2OЃЋCO2Ёќ 2Cu2+ЃЋ4I- = 2CuIЁ§ЃЋI2 ![]()
ЁОНтЮіЁП
ЭЈЙ§ДзЫсЭгыЬМЫсФЦЗДгІжЦЕУМюЪНЬМЫсЭЃЌМюЪНЬМЫсЭдйгыЫсЗДгІЕУЕНДзЫсЭШмвКЃЌОЙ§еєЗЂХЈЫѕЁЂРфШДНсОЇЁЂЙ§ТЫЕУЕНДзЫсЭОЇЬхЁЃЭЈЙ§ЕЮЖЈЗЈВтЖЈВњЦЗжаЭЕФКЌСПЁЃ
(1)ЬМЫсИљРызгЮЊШѕЫсИљРызгЃЌдкЫЎШмвКжаЗЂЩњЫЎНтЗДгІ![]() +H2OOH-+
+H2OOH-+![]() ЃЌЂйжагаOHЩњГЩЃЌЙЪБОЬтД№АИЮЊЃК
ЃЌЂйжагаOHЩњГЩЃЌЙЪБОЬтД№АИЮЊЃК![]() +H2OOH-+
+H2OOH-+![]() ЃЛ
ЃЛ
(2)Й§ГЬЂкжаДзЫсгыМюЪНЬМЫсЭЗДгІЩњГЩДзЫсЭЁЂЖўбѕЛЏЬМКЭЫЎЃЌЛЏбЇЗНГЬЪНЪЧCu2(OH)2CO3ЃЋ4CH3COOH=2(CH3COO)2CuЃЋ3H2OЃЋCO2ЁќЃЌЙЪБОЬтД№АИЮЊЃКCu2(OH)2CO3ЃЋ4CH3COOH=2(CH3COO)2CuЃЋ3H2OЃЋCO2ЁќЃЛ
(3)iжаДзЫсЭгыЕтЛЏМиЗДгІЩњГЩЕтЛЏбЧЭГСЕэКЭДзЫсМиЃЌДзЫсЭЁЂЕтЛЏМиКЭДзЫсМиЖМЪЧПЩШмадбЮЃЌдкРызгЗНГЬЪНжаПЩвдВ№ПЊЃЌдђЗДгІЕФРызгЗНГЬЪНЪЧ2Cu2+ЃЋ4I- = 2CuIЁ§ЃЋI2ЃЌЙЪБОЬтД№АИЮЊЃК2Cu2+ЃЋ4I- = 2CuIЁ§ЃЋI2ЃЛ
(4)ИљОн2Cu2+ЃЋ4I- = 2CuIЁ§ЃЋI2ЃЌ2![]() +I2ЃН
+I2ЃН![]() +2I-ЃЌПЩжЊ2Cu2+ЁЋI2ЁЋ2
+2I-ЃЌПЩжЊ2Cu2+ЁЋI2ЁЋ2![]() ЃЌn(
ЃЌn(![]() )=n(Cu2+)=bЁСvЁС10-3molЃЌДзЫсЭВњЦЗжаЭдЊЫиЕФжЪСПЗжЪ§ЪЧ
)=n(Cu2+)=bЁСvЁС10-3molЃЌДзЫсЭВњЦЗжаЭдЊЫиЕФжЪСПЗжЪ§ЪЧ![]() =
=![]() ЃЌЙЪБОЬтД№АИЮЊЃК
ЃЌЙЪБОЬтД№АИЮЊЃК![]() ЁЃ
ЁЃ

ЁОЬтФПЁПГЃЮТЯТЃЌгаЙиЮяжЪЕФШмЖШЛ§ШчЯТЃЌЯТСагаЙиЫЕЗЈВЛе§ШЗЕФЪЧ( )
ЮяжЪ | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
Ksp | 4.96ЁС10-9 | 6.82ЁС10-6 | 4.68ЁС10-6 | 5.60ЁС10-12 | 2.80ЁС10-39 |
A.ГЃЮТЯТЃЌГ§ШЅNaClШмвКжаЕФMgCl2дгжЪЃЌбЁгУNaOHШмвКБШNa2CO3ШмвКаЇЙћКУ
B.ГЃЮТЯТЃЌГ§ШЅNaClШмвКжаЕФCaCl2дгжЪЃЌбЁгУNaOHШмвКБШNa2CO3ШмвКаЇЙћКУ
C.ЯђКЌгаMg2+ЁЂFe3+ЕФШмвКжаЕЮМгNaOHШмвКЃЌЕБСНжжГСЕэЙВДцЧвШмвКЕФpH=8ЪБЃЌc(Mg2+)ЃКc(Fe3+)=2.0ЁС10-21
D.НЋЪЪСПЕФCa(OH)2ЙЬЬхШмгк100mLЫЎжаЃЌИеКУДяЕНБЅКЭ[c(Ca2+)=1.0ЁС10-2mol/L]ЃЌШєБЃГжЮТЖШВЛБфЃЌЯђЦфжаМгШы100mL0.012mol/LЕФNaOHЃЌдђИУШмвКБфЮЊВЛБЅКЭШмвК
ЁОЬтФПЁПЂёЁЂвбжЊЃКSiHCl3дкДпЛЏМСзїгУЯТПЩЗЂЩњШчЯТЗДгІЃК
2SiHCl3(g)=SiH2Cl2(g)+SiCl4(g) ІЄH1=+48kJЁЄmol1
3SiH2Cl2(g)=SiH4(g)+2SiHCl3(g) ІЄH2=30kJЁЄmol1
(1)аДГігЩSiHCl3(g)ЗжНтВњЩњSiH4(g)КЭSiCl4(g)ЕФШШЛЏбЇЗНГЬЪНЮЊ__ЁЃ
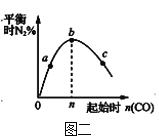
ЂђЁЂ298KЪБЃЌШмвКжаДцдкШчЯТЗДгІЃКA(aq)![]() B(aq)+H2O(l) ІЄH>0ЃЌЮяжЪAЕФГѕЪМХЈЖШЮЊ0.180molЁЄL-1ЃЌВтЕУЮяжЪBЕФХЈЖШЫцЪБМфБфЛЏЕФЪ§ОнШчБэЫљЪОЃК
B(aq)+H2O(l) ІЄH>0ЃЌЮяжЪAЕФГѕЪМХЈЖШЮЊ0.180molЁЄL-1ЃЌВтЕУЮяжЪBЕФХЈЖШЫцЪБМфБфЛЏЕФЪ§ОнШчБэЫљЪОЃК
t/min | 0 | 21 | 50 | 80 | 100 | 120 | 160 | 220 |
|
c/(molЁЄLЃ1) | 0 | 0.024 | 0.050 | 0.071 | 0.081 | 0.090 | 0.104 | 0.116 | 0.132 |
ЛиД№ЯТСаЮЪЬтЃК
(2)ИУЗДгІдк50ЁЋ80minФкЕФЦНОљЗДгІЫйТЪЮЊ__molЁЄL1ЁЄmin1ЁЃ
(3)120minЪБAЕФзЊЛЏТЪЮЊ__ЁЃ
(4)298KЪБЃЌИУЗДгІЕФЦНКтГЃЪ§K=__ЃЌЩ§ЮТЪБЦНКтГЃЪ§__(ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЁЂЛђЁАВЛБфЁБ)ЁЃ
(5)ЮЊЬсИпAЕФЦНКтзЊЛЏТЪЃЌГ§ЪЪЕБПижЦЗДгІЮТЖШЭтЃЌЛЙПЩВЩШЁЕФДыЪЉЪЧ__ЁЃ
(6)ЯТСаа№ЪіПЩЫЕУїПЩФцЗДгІвбДяЦНКтЕФЪЧ__ЁЃ
A.c(A)=c(B)
B.AКЭBЗДгІЫйТЪЯрЕШ
C.AКЭBЕФЮяжЪЕФСПБШжЕБЃГжКуЖЈ
D.ШмвКЕФзмжЪСПБЃГжКуЖЈ