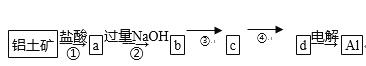
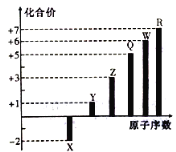
äãá¢áÖàï
Àƒäãá¢À¢ùí¤üüÐÇ¥¢èØåÆûæ¼ò°ÆûüЃ¨È˜óð§Ã¿¿¥·ò§àÓë¥1ùªòƒÈÛ
ØîøˆÈ¤R-CH=CH2 ![]() R-CH2CH2OH
R-CH2CH2OH
![]() أ
Ø£![]() ùí¤üüÐÇ¥çáñøæÆò§öˆ ______ Șù■ý£áÉñÂèºçáÆÅ£ºñÇÆÎâÁÅëÆÅ
ùí¤üüÐÇ¥çáñøæÆò§öˆ ______ Șù■ý£áÉñÂèºçáÆÅ£ºñÇÆÎâÁÅëÆÅ![]() äŸò»æøÅ·¤é
äŸò»æøÅ·¤é![]() ______ ÈÛ
______ ÈÛ
![]() àÀǺñÇÆÎ
àÀǺñÇÆÎ![]() ¥Æ°èñÇÆÎ
¥Æ°èñÇÆÎ![]() ü«àËñÇÆÎ
ü«àËñÇÆÎ![]() ¥ÆƒÜñÇÆÎ
¥ÆƒÜñÇÆÎ![]() î¾£₤ñÇÆÎ
î¾£₤ñÇÆÎ![]() ùÛ§ãñÇÆÎȘ
ùÛ§ãñÇÆÎȘ
![]() ѱ
ѱ![]() ÆÅ£ºöÿÝ«òúØ£øøüÐêüȘóð¤ü°èôñüÔàÓë¥
ÆÅ£ºöÿÝ«òúØ£øøüÐêüȘóð¤ü°èôñüÔàÓë¥![]() óðøÅ¥æçáüÁÑåñøæÆøòê¢ë´¿»øòóæñ´ýãçûöˆ88Șù■çá¤ùÇé¿ýíþúãóæüåòƒø£ÆÅà»æÕñÍÈ£ØØÆŠùí¤üüÐÇ¥£Ëöˆë˜üçöÿÈÛ
óðøÅ¥æçáüÁÑåñøæÆøòê¢ë´¿»øòóæñ´ýãçûöˆ88Șù■çá¤ùÇé¿ýíþúãóæüåòƒø£ÆÅà»æÕñÍÈ£ØØÆŠùí¤üüÐÇ¥£Ëöˆë˜üçöÿÈÛ
![]() ¯Çííüçë°û■û«ñ´È˜Açáû«°óòú ______ ÈÛ
¯Çííüçë°û■û«ñ´È˜Açáû«°óòú ______ ÈÛ
![]() ÆŠÅôøó
ÆŠÅôøó![]() Å■æúؤñÇÆÎçá£₤îÏñ§°äò§öˆ ______ ÈÛ
Å■æúؤñÇÆÎçá£₤îÏñ§°äò§öˆ ______ ÈÛ
![]() Ý«øŤ˜ÆÅꧡ—
Ý«øŤ˜ÆÅꧡ—![]() ȘD¢èñÂèºØ½ƒçñÇÆÎȘåÖÇÔ£₤¥êÇÌåÖüô1molDÆŠ2mol
ȘD¢èñÂèºØ½ƒçñÇÆÎȘåÖÇÔ£₤¥êÇÌåÖüô1molDÆŠ2mol![]() ¢èØåñÇÆÎ躰èØØȘå·Dçá§Ã¿¿¥·ò§öˆ ______ ÈÛ
¢èØåñÇÆÎ躰èØØȘå·Dçá§Ã¿¿¥·ò§öˆ ______ ÈÛ
![]() ¥æÆŠØØñÇÆÎçá£₤îÏñ§°äò§öˆ ______ ÈÛ
¥æÆŠØØñÇÆÎçá£₤îÏñ§°äò§öˆ ______ ÈÛ
![]() ݧ£ñèüÆÅ3¡—àÀǺ£ª£·¿ìáÉëéȘúØüåà¾ùÃÅåçáØØçáë˜ñøØš¿¿äÍ¿ýÆÅ _____ øøȘóðøÅ3¡—àÀǺ£ª£·¿ìáÉëé£Ëý£üÁêÖçáÆÅ£ºöÿ§Ã¿¿¥·ò§öˆ ______ ÈÛ
ݧ£ñèüÆÅ3¡—àÀǺ£ª£·¿ìáÉëéȘúØüåà¾ùÃÅåçáØØçáë˜ñøØš¿¿äÍ¿ýÆÅ _____ øøȘóðøÅ3¡—àÀǺ£ª£·¿ìáÉëé£Ëý£üÁêÖçáÆÅ£ºöÿ§Ã¿¿¥·ò§öˆ ______ ÈÛ
ÀƒÇÞ¯¡À¢![]()
![]()
![]() ¥æ£ªÝ«üˋ (CH3)2CHCHO+2Cu(OH)2
¥æ£ªÝ«üˋ (CH3)2CHCHO+2Cu(OH)2 ![]() (CH3)2CHCOOH+Cu2OÀ»+2H2O
(CH3)2CHCOOH+Cu2OÀ»+2H2O ![]()
![]()
![]()
![]()
![]()
![]() 10
10 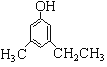
Àƒ§ãö—À¢
(Ø£)¡ªƒïùí¤üüÐÇ¥çá§Ã¿¿¥·ò§ñøö—§ãÇÞÈ£
(ѱ)ØØÆŠùí¤üüÐÇ¥£Ëöˆë˜üçöÿȘØØöˆÇ¥È˜¡ªƒïê¼°äë¥È˜¥æöˆ¶àùÃȘ¥æçáüÁÑåñøæÆøòê¢ë´¿»øòóæñ´ýãçûöˆ88ȘÆèÝ«çáñøæÆò§¢èøˆÈ˜¥æçáñøæÆò§öˆ![]() Ș¥æçá¤ùÇé¿ýíþúãóæüåòƒø£ÆÅà»æÕñÍȘ工æöˆ
Ș¥æçá¤ùÇé¿ýíþúãóæüåòƒø£ÆÅà»æÕñÍȘ工æöˆ![]() ¥æ£ªÝ«ùÃȘ§Ã¤ü¤ü°è戣₤ë¥øÅçáñÇÆÎä¾¥±¢èøˆÈ˜Aöˆ
¥æ£ªÝ«ùÃȘ§Ã¤ü¤ü°è戣₤ë¥øÅçáñÇÆÎä¾¥±¢èøˆÈ˜Aöˆ![]() ¥æ£ªÝ«üˋȘBöˆ
¥æ£ªÝ«üˋȘBöˆ![]() ¥æ£ªÝ«Ç¥È˜Cöˆ
¥æ£ªÝ«Ç¥È˜Cöˆ![]() ¥æ£ªÝ«àˋȘ¥æÆŠØØñÇÆξˣ₤躰èÝ«
¥æ£ªÝ«àˋȘ¥æÆŠØØñÇÆξˣ₤躰èÝ«![]() Șå·ØØöˆ
Șå·ØØöˆ![]() Ș(3)øÅÝ«øŤ˜ÆÅꧡ—
Ș(3)øÅÝ«øŤ˜ÆÅꧡ—![]() ȘD¢èñÂèºØ½ƒçñÇÆÎȘåÖÇÔ£₤¥êÇÌåÖüô
ȘD¢èñÂèºØ½ƒçñÇÆÎȘåÖÇÔ£₤¥êÇÌåÖüô![]() ÆŠ
ÆŠ![]() ¢èØåñÇÆÎ躰èØØȘùªØåDöˆ
¢èØåñÇÆÎ躰èØØȘùªØåDöˆ![]() Șå·Dçá§Ã¿¿¥·ò§öˆ
Șå·Dçá§Ã¿¿¥·ò§öˆ![]() ȘØØöˆ
ȘØØöˆ![]() Șå·Ý«öˆ
Șå·Ý«öˆ![]() ÀȃïÇùñøö—§ãÇÞÀÈ
ÀȃïÇùñøö—§ãÇÞÀÈ
(Ø£)(1)ùí¤üüÐÇ¥(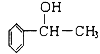 )çáñøæÆò§öˆ
)çáñøæÆò§öˆ![]() Ș§Ã¿¿øŤ˜ÆÅ
Ș§Ã¿¿øŤ˜ÆÅ![]() ¤ëݧ£ñȘ áÉñÂèºàÀǺÀ¥ưèÀÂî¾£₤ÀÂü«àËñÇÆÎȘѽý£áÉñÂèºùÛ§ãÀ¥ƃÜñÇÆÎȘ ¿òÇÞ¯¡öˆÈ¤
¤ëݧ£ñȘ áÉñÂèºàÀǺÀ¥ưèÀÂî¾£₤ÀÂü«àËñÇÆÎȘѽý£áÉñÂèºùÛ§ãÀ¥ƃÜñÇÆÎȘ ¿òÇÞ¯¡öˆÈ¤![]() È£
ȣ![]() ȣ
È£
(ѱ)(2)Æèèüò—ñøö—¢èøˆÈ˜Aöˆ![]() ¥æ£ªÝ«üˋ
¥æ£ªÝ«üˋ![]() £·¥æ£ªÝ«üˋ
£·¥æ£ªÝ«üˋ![]() Ș¿òÇÞ¯¡öˆÈ¤
Ș¿òÇÞ¯¡öˆÈ¤![]() ¥æ£ªÝ«üˋÈ£
¥æ£ªÝ«üˋÈ£
(3)CÆŠÅôøó![]() Å■æúؤñÇÆÎçá£₤îÏñ§°äò§öˆÈ¤(CH3)2CHCHO+2Cu(OH)2
Å■æúؤñÇÆÎçá£₤îÏñ§°äò§öˆÈ¤(CH3)2CHCHO+2Cu(OH)2 ![]() (CH3)2CHCOOH+Cu2OÀ»+2H2O Ș ¿òÇÞ¯¡öˆÈ¤(CH3)2CHCHO+2Cu(OH)2
(CH3)2CHCOOH+Cu2OÀ»+2H2O Ș ¿òÇÞ¯¡öˆÈ¤(CH3)2CHCHO+2Cu(OH)2 ![]() (CH3)2CHCOOH+Cu2OÀ»+2H2OÈ£
(CH3)2CHCOOH+Cu2OÀ»+2H2OÈ£
(4)¥æÆŠØØñÇÆξˣ₤躰èÝ«![]() ȘݫøŤ˜ÆÅꧡ—
ȘݫøŤ˜ÆÅꧡ—![]() ȘåÖÇÔ£₤¥êÇÌåÖüô1molDÆŠ
ȘåÖÇÔ£₤¥êÇÌåÖüô1molDÆŠ![]() ¢èØåñÇÆÎ躰èØØȘDøÅý£¤˜¥æ£ªÈ˜ùªØåDöˆDöˆ
¢èØåñÇÆÎ躰èØØȘDøÅý£¤˜¥æ£ªÈ˜ùªØåDöˆDöˆ![]() Șå·Dçá§Ã¿¿¥·ò§öˆ
Șå·Dçá§Ã¿¿¥·ò§öˆ![]() Ș¿òÇÞ¯¡öˆÈ¤
Ș¿òÇÞ¯¡öˆÈ¤![]() È£
È£
(5)¥æÆŠØØñÇÆÎçá£₤îÏñ§°äò§öˆÈ¤![]()
![]()
![]()
![]()
![]() Ș¿òÇÞ¯¡öˆÈ¤
Ș¿òÇÞ¯¡öˆÈ¤![]()
![]()
![]()
![]()
![]() ȣ
È£
(6)ØØöˆ![]() Ș§Ã¿¿¥·ò§öˆ
Ș§Ã¿¿¥·ò§öˆ![]() Șóðë˜ñøØš¿¿äÍñ«¤üȤݧ£ñèüÆÅ3¡—àÀǺ£ª£·¿ìáÉëéȘüåà¾ùÃÅåȘå·ýÁêÇöˆ
Șóðë˜ñøØš¿¿äÍñ«¤üȤݧ£ñèüÆÅ3¡—àÀǺ£ª£·¿ìáÉëéȘüåà¾ùÃÅåȘå·ýÁêÇöˆ![]() ÀÂ
ÀÂ![]() ÀÂ
ÀÂ![]() Șà¶
Șà¶![]() ÀÂ
ÀÂ![]() üÁêÖȘ
üÁêÖȘ![]() ÆÅ4øøö£øûȘà¶à¶
ÆÅ4øøö£øûȘà¶à¶![]() ÀÂ
ÀÂ![]() üÁ¥ðȘ
üÁ¥ðȘ![]() ÆÅ4øøö£øûȘà¶à¶
ÆÅ4øøö£øûȘà¶à¶![]() ÀÂ
ÀÂ![]() üÁÑåȘ
üÁÑåȘ![]() ÆÅ2øøö£øûȘ¿ò¿ýÆÅ10øøȘóðøÅݧ£ñèü3¡—àÀǺ£ª£·¿ìáÉëé£Ëý£üÁêÖȘ巡ûë˜ñøØš¿¿äÍöˆ
ÆÅ2øøö£øûȘ¿ò¿ýÆÅ10øøȘóðøÅݧ£ñèü3¡—àÀǺ£ª£·¿ìáÉëé£Ëý£üÁêÖȘ巡ûë˜ñøØš¿¿äÍöˆ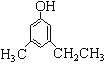 Ș¿òÇÞ¯¡öˆÈ¤10È£
Ș¿òÇÞ¯¡öˆÈ¤10È£ ÀÈ
ÀÈ

 óÔÅúë¥òÕ¢ÖùÐùìùÐäšäšêñüçêÅÇÞ¯¡
óÔÅúë¥òÕ¢ÖùÐùìùÐäšäšêñüçêÅÇÞ¯¡ °¾øÅîÏØ碥òåç¥ÆŠêñüçêÅÇÞ¯¡
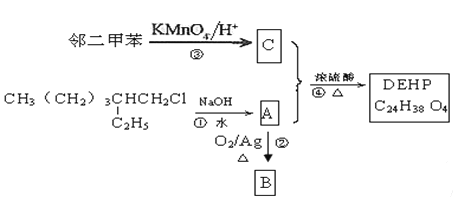
°¾øÅîÏØ碥òåç¥ÆŠêñüçêÅÇÞ¯¡Àƒäãá¢À¢¥æǥȴCH3OHÈˋ¤ëѱ¥æûîÈ´CH3OCH3ÈˋÝ£°óöˆ21òâ¥ëçáÅôÅëà¥êüȘƒÔÆÅúͧÁÀ¡ÔÅÏçàÅåáÉÀÈ
È´1ÈˋCO2¢èÆûÆÖ¤ü°èѱ¥æûîÈ´CH3OCH3ÈˋȘÆÅ¿ÄñÇÆÎçáàà£₤îÏñ§°äò§àÓüôȤ
CO2(g) + 3H2(g)ȧCH3OH(g) + H2O(g) À¼H=ÈÙ49.0 kJÀÊmol-1
2CH3OH(g)ȧCH3OCH3(g) + H2O(g) À¼H=ÈÙ23.5 kJÀÊmol-1
H2O(l)ȧH2O(g) À¼H= + 44 kJÀÊmol-1
å·CO2ÆŠH2ñÇÆΤü°èѱ¥æûî躰èؤä˜ùÛçáàà£₤îÏñ§°äò§öˆÈ¤____________________ÀÈ
È´2Èˋ¿ÊØçèü¤ü°è¥æÇ¥çáñÇÆÎȤCO(g)Ȩ2H2(g)![]() CH3OH(g) À¼H=ÈÙ90.8 kJÀÊmol-1ÀÈ üôêÅý£áÉùçû¼¡ûñÇÆÎåÖ¤Ðöô¤Ðàïä¾¥±üôØîÇÿ£₤îÏó§¤ãæÇä˜çáòú___________
CH3OH(g) À¼H=ÈÙ90.8 kJÀÊmol-1ÀÈ üôêÅý£áÉùçû¼¡ûñÇÆÎåÖ¤Ðöô¤Ðàïä¾¥±üôØîÇÿ£₤îÏó§¤ãæÇä˜çáòú___________
AÈÛví»(H2) = 2váÌ(CH3OH) BÈÛn(CO):n(H2):n(CH3OH)=1:2:1
CÈÛ£š¤üó½äÍçáûÉÑàý£Ýð DÈÛ£š¤üó½äÍçá󧃪üÁÑåñøæÆøòê¢ý£Ýð EÈÛàïó¼çáî¿ú¢ý£Ýð
È´3Èˋà¶ñÇÆÎ2CH3OH(g) ![]() CH3OCH3(g) + H2O(g)åÖá°öôÑàüôçá£₤îÏó§¤ã°Èò»öˆ400ȘÇùöôÑàüôȘåÖûÉÝíàïó¼øÅ¥ÆàŠØ£Ñ´ê¢¥æǥȘñÇÆΧ½ÅÅç§á°òÝ¢äȘýãçû¡¼öÿøòçáé´ÑààÓüôÝÚùªòƒÈ¤
CH3OCH3(g) + H2O(g)åÖá°öôÑàüôçá£₤îÏó§¤ã°Èò»öˆ400ȘÇùöôÑàüôȘåÖûÉÝíàïó¼øÅ¥ÆàŠØ£Ñ´ê¢¥æǥȘñÇÆΧ½ÅÅç§á°òÝ¢äȘýãçû¡¼öÿøòçáé´ÑààÓüôÝÚùªòƒÈ¤
öÿøò | CH3OH(g) | CH3OCH3(g) | H2O(g) |
é´ÑàÈ´molÀÊL-1Èˋ | 0.44 | 0.60 | 0.60 |
ÂìÝà§ü¡ûòÝ¢äí»ÀÂáÌñÇÆÎùìôòçáǵÅÀȤv(í»)_____v(áÌ)È´äŸÀ¯>ÀÝÀÂÀ¯<ÀÝ£·À¯=ÀÝÈˋÀÈ
ÂÖඥÆàŠ¥æÇ¥¤µÈ˜ƒÙ10 minñÇÆÎÇÿç§ó§¤ãȘå·ó§¤ã¤µc(CH3OH)=______________Ș
¡ûòÝ¥ðáÖñÇÆÎùìôòv(CH3OCH3)=_____________ÀÈ
È´4Èˋâ«Æûѱ¥æûîÈ´CH3OCH3ÈˋèÒ¥óØ£¡—à¥êüçÓ°ÄȘÆûKOHàÉؤæ¼çÓ§ãøòàÉؤȘò₤á¨æ—çÓ¥¨È˜¡ûçÓ°Ä¡¤¥¨çÓ¥¨ñÇÆÎò§öˆ___________________________ÀÈØåÇùà¥êüçÓ°Äæ¼öˆëã§ÆçÓåǯÇàÓë¥ùªòƒçÓ§ãê·ùÃëÙàÉؤȘàÓ¿«óÞò¥òÝòÂÆÅ1000mLpH=5çáê·ùÃëÙàÉؤȴ25ÀÌȘCuSO4æÐê¢ÈˋȘأÑöòÝ¥ð¤µàÉؤçápHÝðöˆ1Șà¶Øˆò¿àÉؤ£ø¡Çç§óÞò¥é´ÑàÈ´öôÑàý£ÝðȘ¤—ôåàÉؤäÍ£»çáÝð£₤ÈˋȘ¢èü·àÉؤøÅ¥ÆàŠ______óðøòê¢å¥öˆ_____gÀÈ
Àƒäãá¢À¢AÀÂBÀÂCÀÂDÀÂEƒªöˆÑäøÉóÖåˆùÄȘåÙæÆÅ·ò»ØâÇöå—ǵȘúŠ¡ªƒïÝÚøÅÅéü£ÄÇÞüôêÅöòäãȤ
åˆùÄ | åˆùÄÅåøò£·§Ã¿¿ |
A | æŸëãýÐçÓæÆò»òúóðáÖýÐçÓæÆò»çá2ÝÑ |
B | BåˆùÄçáçËøòåÖ¢íó½øŤ˜ê¢æŸÑÁ |
C | CåˆùÄåÖçÄ¢úøŤ˜ê¢æŸÑÁ |
D | DåˆùÄåÖë˜øÉóÖøŧÞò¶ÅåæŸú¢ |
E | °Èöô°Èî¿üôȘEåˆùÄÅö°èçáçËøòòúçÙ£ó訿ääÍȘ°ÈåÖ£Þ觢֡§§■°ê£» |
È´1ÈˋEåÖåˆùÄøÉóÖÝÚøÅçáö£øû___ÈÛ
È´2ÈˋB查·çËó½ä˜úã£₤öÿçáçÓæÆò§___Șò¶ÆÖ____£₤¤üöÿÈ´äŸÀ¯âŠæÆÀÝ£·À¯¿ý¥ÜÀÝÈˋÈ£Dçá柡ԥÜî¾£₤öÿçáùÛ£₤öÿçÓæÆò§___Șùª¤˜£₤îÏ¥■âÁÅëȤ___ÈÛ
È´3ÈˋBÀÂCÀÂDÀÂE¥·çËâŠæƯŠƒÑÆèǵç§ÅÀù°Å·öˆÈ¤ ____È´äŸâŠæÆñ«¤éÈˋÈÛ
È´4ÈˋÆûçÓæÆò§ÝÚòƒEçáúã£₤öÿçáÅö°è¿»°ä_____ÈÛ
È´5ÈˋÆèAÀÂBÀÂCÆŠúãåˆùÄæÕ°èçáØ£øø°È¥«ùÃò§îöÆŠ¿»ê¢Dçá柡ԥÜî¾£₤öÿçáùÛ£₤öÿñÇÆÎçáâŠæÆñ§°äò§È¤___ÈÛ
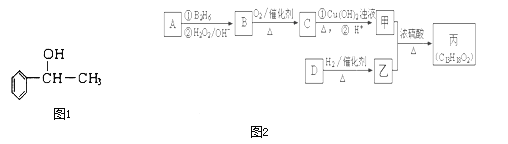
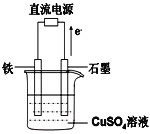
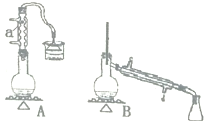
Àƒäãá¢À¢òçîÕòØÆû¥Æàà1Ø£ÑÀÇ¥ÀÂé´H2SO4¤ëðÍ£₤á󣚤üöÿçáñ§ñ´âÇøóÝ¡1Ø£ðÍÑÀëÕȘèÒ¥óêùàÓë¥ùªòƒçáòçîÕæ¯øû![]() óðøÅçá¥Å°øØúó¼ØîòÀôå
óðøÅçá¥Å°øØúó¼ØîòÀôå![]() ÀÈ
ÀÈ
ØîøˆÈ¤H2SO4ȨNaBr=NaHSO4ȨHBrȘ H2SO4È´é´ÈˋȨ2HBr=Br2ȨSO2À■Ȩ2H2O
úŠ£ÄÇÞüôêÅöòäãȤ
(1)Øúó¼açáû«°óöˆ______ÀÈ
(2)øóÝ¡ýìæ¼øÅȘ¥ÆàŠçáé´ê·ùÃòôüà؈§½ÅÅüÀòëȘóðá¢çáòú______![]() äŸîÀüŸæøá¡
äŸîÀüŸæøá¡![]() ÀÈ
ÀÈ
![]() ¥¾èì¡Ýýºöÿüˋ¤ëûîçá躰è
¥¾èì¡Ýýºöÿüˋ¤ëûîçá躰è![]() ¥¾èì
¥¾èì![]() çá躰è
çá躰è![]() ùÛòúñÇÆÎçáÇÔ£₤¥ê
ùÛòúñÇÆÎçáÇÔ£₤¥ê
(3)ÅÇ°—ÇùòçîÕøó1Ø£ðÍÑÀëÕçáæÉ£₤îÏñ§°äò§______ÀÈ
(4)ÆÅë˜îÏáãë´¿»¤šëã¿ãóæØú¥½Ñ´ùªçûýºöÿøÅòúñþ¤˜ÆÅÀ¯![]() ÀÝȘâÇàñÑ´¡ÝýºöÿøÅòúñþÇÌåÖÑÀûî
ÀÝȘâÇàñÑ´¡ÝýºöÿøÅòúñþÇÌåÖÑÀûî![]() úŠóâ¥Ü¡ûë˜îÏèÒ¥óç᥽Ѵñ§¯¡òúñþ¤üâÚÈ¢âÚÆèòú______ÀÈ
úŠóâ¥Ü¡ûë˜îÏèÒ¥óç᥽Ѵñ§¯¡òúñþ¤üâÚÈ¢âÚÆèòú______ÀÈ
(5)öˆêù§½Ø£ý§äÃÇ¢1Ø£ðÍÑÀëÕȘ¡ûÅÀæÕë˜îÏýÕçûüÁ¿ÄÆÅ£ºöÿçáÆÅ¿Äò»ƒïàÓÝÚȤ
öÿøò | àÜçÐ | ñÅçÐ |
1Ø£ÑÀÇ¥ |
|
|
1Ø£ðÍÑÀëÕ |
|
|
ÑÀûî |
|
|
1Ø£ÑÀüˋ |
|
|
å·ÆûBæ¯øûëõ°èÇùäÃÇ¢òçîÕòÝȣȘòçîÕøÅ؈î¡ùì軡ÔöôÑàøê______òí¥₤ùªçûêµñøÀÈ
(6)à¶òçîÕøÅùªàÀ1Ø£ÑÀÇ¥ÀÂNaBrñøÝÞöˆ![]() ÀÂ
ÀÂ![]() Șé´ê·ùÃ
Șé´ê·ùÃ![]() È˜í¶°—çáÇøýºöÿƒÙüÇçÆȘ¡èåÿ¤µåìÇöí¶êµçûç§
È˜í¶°—çáÇøýºöÿƒÙüÇçÆȘ¡èåÿ¤µåìÇöí¶êµçûç§![]() Ø£ðÍÑÀëÕȘå·1Ø£ðÍÑÀëÕçáýºôòòú______
Ø£ðÍÑÀëÕȘå·1Ø£ðÍÑÀëÕçáýºôòòú______![]() ÝÈê¶2ö£ÆÅÅÏò»æø
ÝÈê¶2ö£ÆÅÅÏò»æø![]() ÀÈ
ÀÈ