��Ŀ����
10��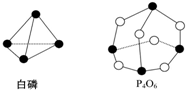 ��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ���Ͽ���1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ•mol-1����P-P��198��P-O��360��O=O��498����1mol��������Ӧ��P4�����ף�+3O2�TP4O6����ЧӦΪ��������
��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ���Ͽ���1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ•mol-1����P-P��198��P-O��360��O=O��498����1mol��������Ӧ��P4�����ף�+3O2�TP4O6����ЧӦΪ��������| A�� | �ų�1 638 kJ������ | B�� | ����1 638 kJ������ | ||
| C�� | �ų�126 kJ������ | D�� | ����126 kJ������ |
���� ��Ӧ�ȡ�H=��Ӧ���ܼ���-�������ܼ��ܣ��ݴ˼����жϣ�ע��ÿĦ��P4�к���6molP-P����
��� �⣺����ѧ������ΪP-P 198kJ•mol-1��P-O 360kJ•mol-1��O=O 498 kJ•mol-1��
��Ӧ�ȡ�H=��Ӧ���ܼ���-�������ܼ��ܣ���ͼ��֪��1��P�����к���6��P-P��1��P4O6�����к���12��P-O��1mol P4��3mol O2��ȫ��Ӧ��P4+3O2=P4O6������1molP4O6�����Է�ӦP4+3O2=P4O6�ķ�Ӧ�ȡ�H=6��198kJ•mol-1+3��498kJ•mol-1-12��360kJ•mol-1=-1638kJ•mol-1����Ӧ����1638kJ��
��ѡ��A��
���� ���⿼�鷴Ӧ���뻯ѧ�����ܵĹ�ϵ���Ѷ��еȣ�ע��������������������ⷴӦ�ȣ�

��ϰ��ϵ�д�
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
�����Ŀ
20��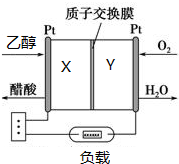 �����Ҵ��������������Ƕ��Ҵ�ȼ�ϵ�ص��о���������Ȥ����ͼ��ʾΪij���Ҵ�ȼ�ϵ�صĹ���ԭ��������˵����ȷ���ǣ�������
�����Ҵ��������������Ƕ��Ҵ�ȼ�ϵ�ص��о���������Ȥ����ͼ��ʾΪij���Ҵ�ȼ�ϵ�صĹ���ԭ��������˵����ȷ���ǣ�������
 �����Ҵ��������������Ƕ��Ҵ�ȼ�ϵ�ص��о���������Ȥ����ͼ��ʾΪij���Ҵ�ȼ�ϵ�صĹ���ԭ��������˵����ȷ���ǣ�������
�����Ҵ��������������Ƕ��Ҵ�ȼ�ϵ�ص��о���������Ȥ����ͼ��ʾΪij���Ҵ�ȼ�ϵ�صĹ���ԭ��������˵����ȷ���ǣ�������| A�� | Y���ĵ缫��ӦʽΪO2+4e-+2H2O�T4OH- | |
| B�� | ��طŵ�ʱ��H+��Y��������X���� | |
| C�� | ÿ����1mol�Ҵ���ת�Ƶ���4mol | |
| D�� | �øõ������Դ��⾫��ͭ��X��Ӧ���ͭ���� |
1��NH3�ʹ�����O2��һ�������·�����Ӧ��4NH3��g��+3O2��g��?2N2��g��+6H2O��g������һ�ݻ������2L�ܱ������г���4mol NH3��3mol O2��4min������ɵ�H2Oռ������������40%�������б�ʾ�˶�ʱ���ڸ÷�Ӧ��ƽ�����ʵ�ʽ�ӣ���ȷ���ǣ�������
| A�� | v��N2���T0.225 mol/��L•min�� | B�� | v��H2O���T0.375 mol/��L•min�� | ||
| C�� | v��O2���T0.225 mol/��L•min�� | D�� | v��NH3���T0.450 mol/��L•min�� |
5������װ������ԭ��ص��ǣ�������
| A�� | 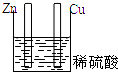 | B�� | 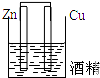 | C�� | 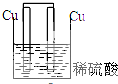 | D�� | 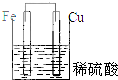 |
2��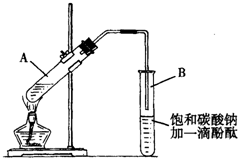 ��֪�������ݣ�
��֪�������ݣ�
ѧ����ʵ������ȡ������������Ҫ�������£�
����30mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10min��
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㣮
�ܷ�������������㡢ϴ�ӡ����
�������ĿҪ��ش��������⣺
��1��д����ȡ���������Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��2������ʵ���б���̼������Һ�������ǣ�����ĸ����BC
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
��3�����������ҪС����ȼ��Ȳ���������Ҫ�����ǣ����������Ҵ��Ļӷ������ٸ���Ӧ�ķ�����
��4��ָ����������۲쵽�������Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz��
 ��֪�������ݣ�
��֪�������ݣ�| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g•cm-3�� |
| �ҡ��� | -117.0 | 78.0 | 0.79 |
| �ҡ��� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���ᣨ98%�� | -- | 338.0 | 1.84 |
����30mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10min��
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㣮
�ܷ�������������㡢ϴ�ӡ����
�������ĿҪ��ش��������⣺
��1��д����ȡ���������Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��2������ʵ���б���̼������Һ�������ǣ�����ĸ����BC
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
��3�����������ҪС����ȼ��Ȳ���������Ҫ�����ǣ����������Ҵ��Ļӷ������ٸ���Ӧ�ķ�����
��4��ָ����������۲쵽�������Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz��
19�����и��������У���Ϊͬ���칹����ǣ�������
| A�� | CH3CH2CH2CH3��CH3CH=CHCH3 | B�� | CH3CH=CHCH3�� CH3C��CCH3 | ||
| C�� | CH3CH=CHCH3��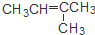 | D�� | CH3CH2CH2OH��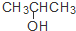 |
20������˵����ȷ���ǣ�������
| A�� |  ��ͼ�ɱ�ʾˮ�ֽ�����е������仯 ��ͼ�ɱ�ʾˮ�ֽ�����е������仯 | |
| B�� | ��2C��s��+O2��g��=2CO��g����H=-221.0 kJ/mol����̼��ȼ����Ϊ110.5 kJ/mol | |
| C�� | ��Ҫ���ȵķ�Ӧһ�������ȷ�Ӧ���������ܷ����ķ�Ӧһ���Ƿ��ȷ�Ӧ | |
| D�� | ��֪�����ڷ�Ӧ��H2��g��+Cl2��s��=2HCl ��g����H=-a kJ/mol�� �� 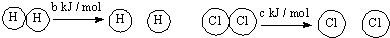 ��a��b��c�������㣬��Ͽ�1 mol H-Cl�����������Ϊ-a-b-c ��a��b��c�������㣬��Ͽ�1 mol H-Cl�����������Ϊ-a-b-c |
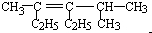 2��4-����-3-�һ�-3-��ϩ
2��4-����-3-�һ�-3-��ϩ 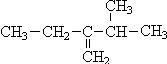 3-��-2-�һ�-1-��ϩ
3-��-2-�һ�-1-��ϩ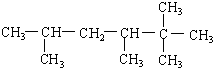 2��2��3��5-�ļ����飮
2��2��3��5-�ļ����飮