��Ŀ����
���¶�T1��T2ʱ���ֱ�0.50 mol CH4��1.2mol NO2����1 L���ܱ������з�����Ӧ��
CH4��g����2NO2��g�� N2��
N2�� g����CO2��g����2H2O��g����H=akJ/mol������й��������±���
g����CO2��g����2H2O��g����H=akJ/mol������й��������±���
�¶� | ʱ��/min ���ʵ��� | 0 | 10 | 20 | 40 | 50 |
T1 | n��CH4��/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
T2 | n��CH4��/mol | 0.50 | 0.30 | 0.18 | x | 0.15 |
����˵����ȷ����
A��T1��T2����a��0
B�����¶�ΪT2����Ӧ���е�40 minʱ�� x��0.15
C���¶�ΪT2ʱ������ƽ�����������ٳ���0.50 mol CH4��1.2mol NO2�����´ﵽƽ��ʱ��n��N2��=0.70mol
D���¶�ΪT1ʱ����ƽ��ʱCH4 ��ת���ʴ���NO2��ת����
| A�� | ��IA�������Ԫ�ض��ǽ���Ԫ�� | |
| B�� | ͬ��������Ԫ���У��ڢ�A��Ԫ��ԭ�Ӱ뾶��С | |
| C�� | ϡ������Ԫ��ԭ�ӵ�������������Ϊ8 | |
| D�� | һ����Ԫ�����ڱ��Ľ�����ǽ����ֽ��߸���Ѱ��һЩ��ѧ��Ӧ���ʹ��� |
ij������ȤС���H2O2�ķֽ�������������ʵ��̽����
��1���±��Ǹ�С���о�Ӱ��������⣨H2O2���ֽ����ʵ�����ʱ�ɼ���һ�����ݣ�
��10 mL H2O2��ȡ150 mL O2�����ʱ��
Ũ�� ʱ�䣨�룩 ��Ӧ���� | 30%H2O2 | 15%H2O2 | 10%H2O2 | 5%H2O2 |
���������������� | ���� ����Ӧ | ���� ����Ӧ | ���� ����Ӧ | ���� ����Ӧ |
�������������� | 360 | 480 | 540 | 720 |
����MnO2���������� | 10 | 25 | 60 | 120 |
��С������Ʒ���ʱ��������Ũ�ȡ�a��___________b��___________�����ضԹ�������ֽ����ʵ�Ӱ�졣
�ڴ�����Ӱ��H2O2�ֽ����ʵ�����a��b����ѡһ����˵�������ضԸ÷�Ӧ���ʵ�Ӱ�죺_____________��
��2����������ͬ��������С��ͬ��MnO2�ֱ���뵽5 mL 5%��˫��ˮ�У����ô����ǵ�ľ�����ԡ��ⶨ������£�
���� ��MnO2�� | ������� | �۲��� | ��Ӧ��������ʱ�� |
��ĩ״ | ��ϲ��� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5���� |
��״ | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30���� |
��д��H2O2������Ӧ�Ļ�ѧ����ʽ��______________________��
��ʵ����˵���������õĴ�С��________________�йء�
| A�� | ��һ�����ܣ��ڣ��ۣ��� | B�� | ԭ�Ӱ뾶���ۣ��ڣ��� | ||
| C�� | �縺�ԣ��٣��ڣ��� | D�� | ��������ϼۣ��ۣ��ڣ��� |


 2NO2��g�� ��H��0 ���¶�ΪTl��T2ʱƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����__________��
2NO2��g�� ��H��0 ���¶�ΪTl��T2ʱƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����__________��
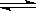 4C��������2D����������Ӧһ��ʱ���ﵽƽ�⣬�������1.6 mol C���ҷ�Ӧ��ǰ��ѹǿ֮��Ϊ5��4����ͬ���¶��²���������÷�Ӧ�Ļ�ѧƽ�ⳣ����K=____________��
4C��������2D����������Ӧһ��ʱ���ﵽƽ�⣬�������1.6 mol C���ҷ�Ӧ��ǰ��ѹǿ֮��Ϊ5��4����ͬ���¶��²���������÷�Ӧ�Ļ�ѧƽ�ⳣ����K=____________�� 4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135��7 kJ/mol
4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135��7 kJ/mol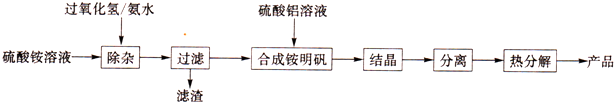
 cC��g������Ӧ�����У�����A�ĺ�����A������C�ĺ�����C�������¶ȣ�T���ı仯������ͼ��ʾ������˵����ȷ����
cC��g������Ӧ�����У�����A�ĺ�����A������C�ĺ�����C�������¶ȣ�T���ı仯������ͼ��ʾ������˵����ȷ����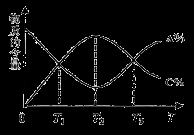
 �����ƶ�
�����ƶ�