��Ŀ����
����Ŀ����Ba(OH)2��Һ����μ���ϡ���ᣬ������������⣺
��1��д����Ӧ�����ӷ���ʽ____________________��
��2��������������£����ӷ���ʽ�루1����ͬ����_____������ţ���
A.��NaHSO4��Һ�У���μ���Ba(OH)2��Һ����Һ������
B.��NaHSO4��Һ�У���μ���Ba(OH)2��Һ��SO42-ǡ����ȫ����
C.��NaHSO4��Һ�У���μ���Ba(OH)2��Һ������
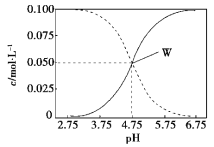
��3������������ϡ����ֱ�����������������л����Һ�ĵ����������õ���ǿ��![]() ��ʾ���ɽ��Ƶ���ͼ�е�_____���߱�ʾ������ţ���
��ʾ���ɽ��Ƶ���ͼ�е�_____���߱�ʾ������ţ���
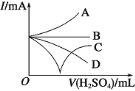
��4������һ����⻬������С��������Ba(OH)2��Һ���룬��ͼ��ʾ������ձ��ﻺ��ע����Ba(OH)2��Һ���ܶȵ�ϡ������ǡ����ȫ��Ӧ���ڴ�ʵ������У�С��_____��ѡ�����ϸ������������������½�������
���𰸡�![]() A C �½�
A C �½�
��������
��1���������������ᷴӦ�������ᱵ������ˮ��
��2��A��NaHSO4��Һ�У���μ���Ba(OH)2��Һ����Һ�����ԣ���Ӧ���������ơ����ᱵ��ˮ��
B����NaHSO4��Һ�У���μ���Ba(OH)2��Һ��SO42-ǡ����ȫ��������Ӧ�������ᱵ���������ƺ�ˮ��
C����NaHSO4��Һ�У���μ���Ba(OH)2��Һ����������Ӧ�������ᱵ���������ƺ�ˮ��
��3������ϡ����ֱ��������ǡ�÷�Ӧʱ������Ϊ0������������Ũ������������ǿ��
��4������С��������Ba(OH)2��Һ���룬˵������С����������ڸ�������ע��ϡ���������Ӧ��Ba2++2OH+2H++SO42�TBaSO4��+2H2O�����ʵ�������С��������Һ���ܶȱ�С�����������ܸ�����С�������ڴ�ʵ������У�С�����½���
��1����Ba(OH)2��Һ����μ���ϡ���ᣬ���ӷ���ʽΪBa2++2OH+2H++SO42�TBaSO4��+2H2O��
�ʴ�Ϊ��Ba2++2OH+2H++SO42�TBaSO4��+2H2O��
��2��A. ��NaHSO4��Һ�У���μ���Ba(OH)2��Һ����Һ�����ԣ���Ӧ�����ӷ���ʽΪ��Ba2++2OH+2H++SO42�TBaSO4��+2H2O��A����ȷ��
B. ��NaHSO4��Һ�У���μ���Ba(OH)2��Һ��SO42ǡ����ȫ��������Ӧ�����ӷ���ʽΪ��Ba2++OH+H++SO42�TBaSO4��+H2O��B�����
C. ��NaHSO4��Һ�У���μ���Ba(OH)2��Һ����������Ӧ�����ӷ���ʽΪ��Ba2++OH+H++SO42=BaSO4��+H2O��C�����
�ʴ�Ϊ��A��
��3������ϡ����ֱ��������ǡ�÷�Ӧʱ������Ϊ0������������Ũ������������ǿ��ͼ��ֻ��C���ϣ�
�ʴ�Ϊ��C��
��4������С��������Ba(OH)2��Һ���룬˵������С����������ڸ�������ע��ϡ���������Ӧ��Ba2++2OH+2H++SO42�TBaSO4��+2H2O�����ʵ�������С��������Һ���ܶȱ�С�����������ܸ�����С�������ڴ�ʵ������У�С�����½���
�ʴ�Ϊ���½���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�