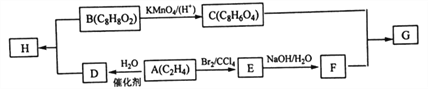
��Ŀ����
����Ŀ������ѧһѡ��3�����ʽṹ�����ʡ�
±��Ԫ�ص������������ʷ�Ӧ�γɶ��ֻ������������ѧ���ʽṹ�����ʵ����֪ʶ�ش�
(1)д����̬��ԭ�ӵļ۵����Ų�ʽ___________��
(2)±��Ԫ�صĺ�������������ǿ����____(д��ѧʽ)����������ӵ����幹��Ϊ_________��
(3)�Ƚ��������±������۵�ͷе㣬������仯���ɼ�ԭ��_________��
GeCl4 | GeBr4 | GeI4 | |
�۵�/�� | -49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
(4)��֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6(![]() )��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
)��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
(5)��֪��Ԫ����������������ֻ��1�����ӡ�����������ԭ�ӹ�����������ӵ�Ԫ��M�γɵ�һ�ֻ����������������ͼ��ʾ��
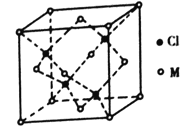
�ٸû�����Ļ�ѧʽΪ_______����֪��������a=0.542 nm���˾�����ܶ�Ϊ_____g/cm3��(д������ʽ����Ҫ��������������ӵ�����ΪNA)
���𰸡� 5s25p3 HClO4 �������� GeCl4��GeBr4��GeI4���ۡ��е��������ߡ�ԭ���Ƿ��ӽṹ���ơ���Է����������������Ӽ������������ǿ < 11:1 CuCl ![]() ��
��![]()
��������(1)��ĺ˵����Ϊ53����̬��ԭ�ӵĵ����Ų�ʽΪ[Kr]4d105s25p5����۵����Ų�ʽ5s25p3��
(2)���ȵ����и�����������ǿ�Ǻ���������ǿ�ᣬ��ѧʽΪ��HClO4�������������������ԭ��ΪsP3�ӻ���û�й¶Ե����������幹��Ϊ�������壻
(3)���±���ﶼ�Ƿ��Ӿ��壬���Ӽ�ͨ�����Ӽ���������ϣ����������ṹ���Ƶķ��Ӿ��壬��Է�������Խ���Ӽ�������Խǿ���۷е�Խ�ߣ�������Է���������GeCl4��GeBr4��GeI4���ʷе㣺GeCl4��GeBr4��GeI4��
(4)H5IO6(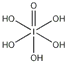 )�к���5���ǻ��⣬Ϊ��Ԫ�ᣬ�����ǻ���ԭ��1����HIO4ΪһԪ�ᣬ����1���ǻ��⣬�����ǻ���ԭ��3�����������ԣ�H5IO6��HIO4��H5IO6��������11��������Ϊ1�����ߵĸ�����Ϊ11:1��
)�к���5���ǻ��⣬Ϊ��Ԫ�ᣬ�����ǻ���ԭ��1����HIO4ΪһԪ�ᣬ����1���ǻ��⣬�����ǻ���ԭ��3�����������ԣ�H5IO6��HIO4��H5IO6��������11��������Ϊ1�����ߵĸ�����Ϊ11:1��
(5)���ݾ����ṹ�������и������ÿ�������к���ͭԭ�Ӹ���Ϊ��8��![]() +6��
+6��![]() =4����ԭ�Ӹ���Ϊ4����ѧʽΪ��CuCl��1mol�����к���4molCuCl��1mol����������ΪM(CuCl)��4����������a=0.542nm�������ܶ�Ϊ
=4����ԭ�Ӹ���Ϊ4����ѧʽΪ��CuCl��1mol�����к���4molCuCl��1mol����������ΪM(CuCl)��4����������a=0.542nm�������ܶ�Ϊ![]() =
=![]() ��
��

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�