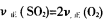��Ŀ����
��1����֪2H2��g��+O2��g��=2H2O��l����H����571.6 kJ/mol��
CO��g����1/2O2��g����CO2��g����H����283.0 kJ/mol��ijH2��CO�Ļ��������ȫȼ��ʱ�ų�113.74 kJ������ͬʱ����3.6 gҺ̬ˮ����ԭ���������H2��CO�����ʵ���֮��Ϊ___________��
��2���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫���ɹ���ȼ�ϵ�أ���֪��ȼ�ϵ�ص��ܷ�Ӧʽ�ǣ�2CH3OH +3O2+4OH-=2CO32-+6H2O����ȼ�ϵ�ط�����Ӧʱ����������Һ��PH__________ (����� ����С�� ���䡱)�õ�صĸ�����ӦʽΪ_________________��
��3�� ������ȼ�ϵ�ؽ��д�ͭ�ľ�������ͭӦ���ӵ�Դ��________�����ô�ͭ�������ص�������ӦʽΪ_________________��
��1��1��1......2��
��2������.1�� CH3OH -6e����8OH-��1����CO32-��6H2O ....2��
��3������..1�֡� Cu2+ + 2e- =Cu��.2��
��������������⣺ˮ�����ʵ���Ϊ=0.2mol����2H2+O2�T2H2O��֪��n��H2��=n��H2O��=0.2mol����2H2��g��+O2��g���T2H2O��l����H=-571.6kJ?mol-1��֪��0.2molH2ȼ�շų�������Ϊ57.16KJ����COȼ�շų�������Ϊ113.74KJ-57.16KJ=56.58KJ������������CO�����ʵ���Ϊx����
CO��g��+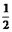 O2��g��=CO2��g����H=-283kJ?mol-1
O2��g��=CO2��g����H=-283kJ?mol-1
1 283KJ
X 56.58KJ
���x=0.2mol����n��CO��=0.20mol����ԭ���������H2��CO�����ʵ���֮��Ϊ1:1.��2���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�أ�������ӦΪ��3O2+12H2O+12e-=12OH-���ܷ�ӦʽΪ��2CH3OH+3O2+4OH-=2CO32-+6H2O����ʽ�����������ӦΪ��2CH3OH-12e-+16OH-=2CO32-+12H2O����3���͵�Դ�����������ĵ缫����������ⷽ��������ͭ�����ص����������Ǵ�ͭ���缫��ӦΪ��Cu-2e-=Cu2+
���㣺�йط�Ӧ�ȵļ��㣻�ø�˹���ɽ����йط�Ӧ�ȵļ��� ��ѧ��Դ���͵��

 ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д���ѧ��Ӧ�仯���̼�������о�����Ҫ��ش����⣺
��1�����ڷ�Ӧ�����������仯���о���
��2CO��g��+O2��g��=2CO2��g����H= kJ��mol-1��
��2�����ڷ�Ӧ���ʺ��ȵ��о���
��ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO(NH2)2]����Ӧ�Ļ�ѧ����ʽΪ��
2NH3 (g)+ CO2 (g)  CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ����K�����¶ȣ�T / �棩��ϵ���£�
CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ����K�����¶ȣ�T / �棩��ϵ���£�
| T / �� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
���ʱ䦤H _______0 ���>������<����=������
����һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ�
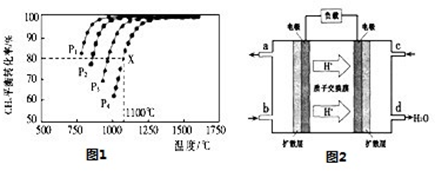
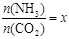 ����ͼ��1���ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��
����ͼ��1���ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��
����ͼ�е�B�㴦��NH3��ƽ��ת����Ϊ ��
��3�����ڵ绯ѧ���о���
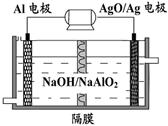
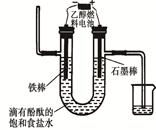
�����ճ���������;���Ľ���Ԫ�أ���ͼΪAl-AgO��صĹ����ͼ���������ҺΪNaOH����������ˮ�¶�����Դ���õ�������缫��ӦʽΪ ���øõ�ص������[CO(NH2)2]�ļ�����Һ�����װ��ʾ��ͼ����ͼ�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ ��

��4�����ڵ���ƽ����о���
����ѪҺ�������Ҫ�����ƽ�⣺
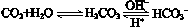 ��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��c(HCO3-)��c(H2CO3)�仯��ϵ���±���
��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��c(HCO3-)��c(H2CO3)�仯��ϵ���±���| c(HCO3-)��c(H2CO3) | 1.0 | 17.8 | 20.0 | 22.4 |
| pH | 6.10 | 7.35 | 7.40 | 7.45 |
�Իش�
��������ѪҺ�У�HCO3-��ˮ��̶� ����̶ȣ�����ڡ�����С�ڡ��������ڡ�����
������ѪҺ���ж�ʱ����ע�仺�� ����ѡ���
A��NaOH��Һ B��NaHCO3��Һ C��NaCl��Һ D��Na2SO4��Һ
�� pH=7.00��ѪҺ�У�c(H2CO3) c(HCO3��) ���<������>������������
����ƽ�ⳣ������Ka��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
| ��ѧʽ | HF | H2CO3 | HClO |
| ����ƽ�ⳣ�� ��Ka�� | 7.2��10-4 | K1=4.4��10-7 K2=4.7��10-11 | 3.0��10-8 |
��1����֪25��ʱ����HF(aq)+OH��(aq)��F��(aq)+H2O(l) ��H����67.7kJ/mol��
��H+(aq)+OH��(aq)��H2O(l) ��H����57.3kJ/mol��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ________________________��
��2����Ũ��Ϊ0.1 mol/LHF��Һ��ˮϡ��һ���������¶Ȳ��䣩�����и����������____��
A��c(H+) B��c(H+)��c(OH��) C��
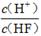 D��
D��
��3��25��ʱ����20mL0.1mol/L������м���VmL0.1mol/LNaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_____��
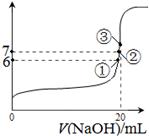
A��pH��3��HF��Һ��pH��11��NaF��Һ�У� ��ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(F��)< c(Na+)��0.1mol/L
��4�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L������������Һ�� �� Na2CO3��Һ �� NaHCO3��Һ�� NaF��Һ ��NaClO��Һ�����������ж�pH�ɴ�С��˳����______________��
��5��Na2CO3��Һ�Լ�������ΪCO32��ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤��֮
___________________________________________________________��
��6������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A��ÿ����1molHFת�� mol���ӡ�
������һ����ࡢ��Ч��������Դ��
I.�ü�����ȡ�����ķ�Ӧ��Ϊ�������������仯����ͼ��ʾ��
��1�������ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ�� ��
II.���ݻ�Ϊ1L���ܱ������ڣ�����0.1molCO��0.1molH2O���ڴ������ڵ������¸��¼���ʹ�䷴Ӧ�����CO2��Ũ����ʱ��仯��ͼ����ͼ��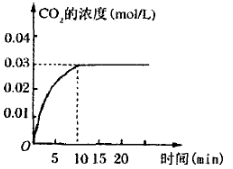
��2���ڸ��¶��£��ӷ�Ӧ��ʼ���ﵽƽ��ʱ��CO��ƽ����Ӧ����Ϊ ��
��3�����¶��£��˷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ�������÷�����ʾ�� ��
��4�����иı��У���ʹƽ��������Ӧ�����ƶ����� ��
| A�������¶� | B������ѹǿ |
| C������H2O��g����Ũ�� | D������CO2��g����Ũ�� |
 CO��g��+3H2��g�����Իش��������⡣
CO��g��+3H2��g�����Իش��������⡣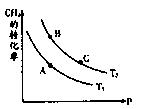
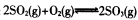 ������ݻ�ѧ��Ӧ���й�ԭ��ͬ����������
������ݻ�ѧ��Ӧ���й�ԭ��ͬ����������