��Ŀ����
ij�¶�(t ��)ʱ��ˮ�����ӻ�ΪKW��1.0��10��13mol2��L��2������¶�(����ڡ�����С�ڡ����ڡ�)________25 �棬��������_______________________________________��
�������¶���pH��11�Ŀ�������Һa L��pH��1��ϡ����b L���(���Ϻ���Һ�����С�仯���Բ���)����ͨ��������д���²�ͬ���ʱ������Һ������ȣ�
(1)�����û��ҺΪ���ԣ���a��b��________������Һ�и������ӵ�Ũ���ɴ�С����˳����___________________________________��
(2)�����û��Һ��pH��2����a��b��________������Һ�и������ӵ�Ũ���ɴ�С����˳����__________________________________________��
�����ڡ�ˮ�ĵ��������ȹ��̣�KW���¶����߶�����(1)10��1��c(Na��)��c(SO)��c(H��)��c(OH��)��c(Na��)��2c(SO42��)��c(H��)��c(OH��)��(2)9��2
c(H��)��c(SO42��)��c(Na��)��c(OH��)
����

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д���ҵ̼����(����ԼΪ98%)�к���Ca2����Mg2����Fe3����Cl����SO42�������ʣ��ᴿ�����������£�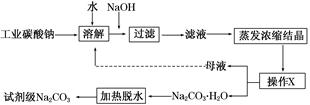
��.̼���Ƶı�����Һ�ڲ�ͬ�¶�����������������ͼ��ʾ��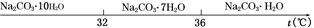
��.�й����ʵ��ܶȻ����£�
| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
�ش��������⣺
(1)����NaOH��Һ����˵õ�����������Ҫ����________(��д��ѧʽ)��25��ʱ������Mg2����Fe3������Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH��8 ʱ��c(Mg2��)��c(Fe3��)��________��
(2)����XΪ________�����¶�Ӧ������_____________________________________
(3)���˴ӡ���ɫ��ѧ���Ƕ����뽫��ĸҺ����������������ʾ����ѭ��ʹ�á��������ʵ�ʹ�ҵ�������Ƿ����________����˵������______________________________
________________________________________________________________________��
�����£���ijһԪ��HA(�ס��ҡ�������������ͬ��һԪ��)��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH���±���ʾ��
| ʵ�� ��� | HA���ʵ��� Ũ��/(mol��L��1) | NaOH���ʵ��� Ũ��/(mol��L��1) | ��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH��a |
| �� | 0.12 | 0.1 | pH��7 |
| �� | 0.2 | 0.1 | pH>7 |
| �� | 0.1 | 0.1 | pH��10 |
��2����������Һ������Ũ��c(A��)��c(Na��)�Ĵ�С��ϵ ��
A��ǰ�ߴ� B�����ߴ� C��������� D�����ж�
��3���ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����________________��
��4����������ʵ�����ݣ�д�������Һ��������ʽ�ľ�ȷ���(ֻ��ʽ)��c(Na��)��c(A��)��________mol��L��1��
��ij��Ԫ��(��ѧʽ��H2B��ʾ)��ˮ�еĵ��뷽��ʽ�ǣ�H2B=H����HB����HB��
 H����B2��
H����B2���ش��������⣺
��5����0.1 mol��L��1��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����
A��c(B2��)��c(HB��)��0.1 mol��L��1
B��c(B2��)��c(HB��)��c(H2B)��0.1 mol��L��1
C��c(OH��)��c(H��)��c(HB��)
D��c(Na��)��c(H��)��c(OH��)��c(HB��)
��֪ij��Һ��ֻ����OH����H+��NH4+��Cl���������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ��
| A��c(NH4+)��c(Cl��)��c(OH��)��c(H+) | B��c(Cl��)��c(H+)��c(NH4+)��c(OH��) |
| C��c(Cl��)��c(NH4+)��c(H+)��c(OH��) | D��c(NH4+)��c(OH��)��c(Cl��)��c(H+) |
�� ��ΪHCl ��NH4Cl �����Һ������������Ũ�ȵ��ɴ�С��˳����_________��
��ΪNH3�� H2O ��NH4Cl �����Һ����������Ũ�ȵ��ɴ�С��˳����__________��
�� ������Һ���������ȵ�HCl��Һ�Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰc(HCl) _____c(NH3��H2O)���>������<������=������ͬ������Ϻ���Һ��c(OH��)____c(NH4+)��

 H����A2����
H����A2���� H����B2�����ش��������⡣
H����B2�����ش��������⡣