��Ŀ����
�����£���ijһԪ��HA(�ס��ҡ�������������ͬ��һԪ��)��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH���±���ʾ��
| ʵ�� ��� | HA���ʵ��� Ũ��/(mol��L��1) | NaOH���ʵ��� Ũ��/(mol��L��1) | ��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH��a |
| �� | 0.12 | 0.1 | pH��7 |
| �� | 0.2 | 0.1 | pH>7 |
| �� | 0.1 | 0.1 | pH��10 |
��2����������Һ������Ũ��c(A��)��c(Na��)�Ĵ�С��ϵ ��
A��ǰ�ߴ� B�����ߴ� C��������� D�����ж�
��3���ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����________________��
��4����������ʵ�����ݣ�д�������Һ��������ʽ�ľ�ȷ���(ֻ��ʽ)��c(Na��)��c(A��)��________mol��L��1��
��ij��Ԫ��(��ѧʽ��H2B��ʾ)��ˮ�еĵ��뷽��ʽ�ǣ�H2B=H����HB����HB��
 H����B2��
H����B2���ش��������⣺
��5����0.1 mol��L��1��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����
A��c(B2��)��c(HB��)��0.1 mol��L��1
B��c(B2��)��c(HB��)��c(H2B)��0.1 mol��L��1
C��c(OH��)��c(H��)��c(HB��)
D��c(Na��)��c(H��)��c(OH��)��c(HB��)
��1��a��7ʱ��HA��ǿ� a>7ʱ��HA������
��2��C
��3��c(Na��)>c(A��)>c(OH��)>c(H��)
��4��10��4��10��10
��5��AC
���������������1��������ͬŨ�ȵ������������ϣ��������ɵ��ε�ˮ���������ˣ�a��7ʱ��HA��ǿ�a>7ʱ��HA�����ᡣ
��2��������غ��У�c(Na��)��c(H��)��c(OH��)��c(A��)����ΪpH��7�����ԣ�c(Na��)��c(A��)��ѡC ��
��3����������Ϊ�ǵ�Ũ�ȵ�HA��NaA�Ļ�ϣ�HA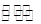 H����A��
H����A��
A����H2O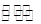 HA��OH��
HA��OH��
����Ϊ�Լ��ԣ�����ˮ��̶ȱȵ���̶ȴ������У�c(Na��)>c(A��)>c(OH��)>c(H��)
��4�� ������غ��У�c(Na��)��c(H��)��c(OH��)��c(A��)��
��ˣ�c(Na��)��c(A��)��c(OH��)��c(H��)��10��4��10��10��
��5��A�������غ㣬��ȷ��B����Һ�в�����H2B ������C�������غ㣬��ȷ��D�����ݵ���غ������¹�ϵʽ��c(Na��)��c(H��)��c(OH��)��c(HB��)��2c(B2��)���������ѡAC��
���㣺���������ˮ���������ʵĵ��롣

 �������ϵ�д�
�������ϵ�д�������,��ijһԪ��HA(�ס��ҡ�������������ͬ��һԪ��)��NaOH��Һ��������,������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH�����ʾ:
| ʵ���� | HA�����ʵ� ��Ũ��(mol��L-1) | NaOH�����ʵ� ��Ũ��(mol��L-1) | ��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH>7 |
| �� | 0.1 | 0.1 | pH=10 |
(1)�Ӽ����������,����ж�HA��ǿ�ỹ������?
(2)��������Һ������Ũ��c(A-)��c(Na+)�Ĵ�С��ϵ�������� ����
A.ǰ�ߴ� B.���ߴ� C.������� D.���ж�
(3)�ӱ���ʵ��������,�û����Һ������Ũ���ɴ�С��˳������ ��
(4)��������ʵ������,д���û����Һ��������ʽ�ľ�ȷ���(��ʽ):
c(Na+)-c(A-)=����������������mol��L-1��
��1������������ ��Cu����Һ̬SO2����CH3COOH����NaHCO3����H2O��������NaCl����BaSO4 ����������ʵ��� ������ţ�
��2�������£�0.1 mol��L-1NaHCO3��Һ��pH����8������Һ��Na����HCO3�D��CO32�D��OH�D��������Ũ���ɴ�С��˳��Ϊ�� ��NaHCO3ˮ������ӷ���ʽ ��
��3�������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HA���ʵ���Ũ��(mol��L-1) | NaOH���ʵ���Ũ��(mol��L-1) | �����Һ ��pH |
| a | 0.1 | 0.1 | pH��9 |
| b | c | 0.2 | pH��7 |
��ش�
�ٴ�a����������� HA��ǿ�ỹ������ ��
��b�����������c 0.2 (ѡ����ڡ�����С�ڡ��� �����ڡ�)�������Һ������Ũ��c(A��)_______ c(Na��)��(ѡ����ڡ�����С�ڡ��� �����ڡ�)
�� a��ʵ�����û����Һ����ˮ�������c(OH��)�� mol��L��1��
ijͬѧ�ù�ҵ����ͭ�����������������ʣ��Ʊ�������CuSO4��5H2O��������������
�����ֲ����������ԣ���
I��ȡ��ҵ����ͭ���壬��ϡ�����ܽ⣬���ˡ�
II������Һ�еμ�H2O2��Һ���Լ��ȡ�
III����II����Һ�м���CuO��ĩ��pH��4��
IV��������У����ˣ���Һ��ϡ�����ữ��pH��1��
V������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����þ��塣
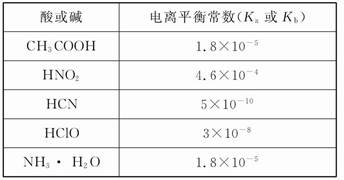
��֪�����������������������pH��Ksp��25�棩���±���
| ���� | Fe(OH)3 | Fe (OH)2 | Cu(OH)2 |
| ��ʼ����ʱpH | 2.7 | 7.6 | 4.7 |
| ��ȫ����ʱpH | 3.7 | 9.6 | 6.7 |
| Ksp | 4.0��10�C38 | 8.0��10�C16 | 2.2��10�C20 |
��1��II�з�����Ӧ�����ӷ���ʽ�� ��
��2��II�н�Fe2+����ΪFe3+��Ŀ���� ��
��3����K3[Fe(CN)6]�����軯�أ���֤II��Fe2+�Ƿ�ת����ȫ�������� ��
��4��III�з�����Ӧ�����ӷ���ʽ�� ��
ͨ������˵���ڴ������µ���Һ��Fe3+�Ƿ������ȫ________________________(��ʾ������Һ��ij����Ũ��С��1.0��10�C5 mol/Lʱ����Ϊ�����ӳ�����ȫ)��
��5��Ӧ�û�ѧƽ���ƶ�ԭ������IV�С���Һ��ϡ�����ữ����ԭ�� ��
ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ�顣
̽��Ũ�ȶԴ������̶ȵ�Ӱ��
��pH�Ʋⶨ25��ʱ��ͬŨ�ȵĴ����pH���������£�
| ����Ũ��(mol/L) | 0.0010 | 0.0100 | 0.0200 | 0.1000 | 0.2000 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
�ش��������⣺
��1��д������ĵ��뷽��ʽ�� ��
��2��������Һ�д��ڵ����� ��
��3�����ݱ������ݣ����Եó�������������ʵĽ��ۣ�����Ϊ�ó��˽��۵������� ��
��4���ӱ��е����ݣ������Եó���һ���ۣ����Ŵ���Ũ�ȵļ�С������ĵ���̶ȣ�������С�䣩 ��
 CH3COO��+H+ ��H��0
CH3COO��+H+ ��H��0

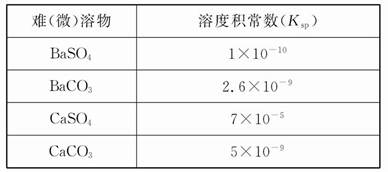
 mMn+��aq�� + nAm����aq����Ksp��[c��Mn+��]m��[c��Am����]n��
mMn+��aq�� + nAm����aq����Ksp��[c��Mn+��]m��[c��Am����]n��